Abstract
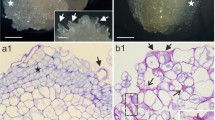
Monoclonal antibodies (2F4), specific for a conformational epitope of homopolygalacturonic acid induced by calcium ions, were used to compare the nature and the distribution of the pectic polysaccharides in cell walls of compact and friable sugar-beet (Beta vulgaris L. var. altissima) calli, at the electron-microscope level. Labelings performed before or after de-esterification pretreatments of callus sections enabled three major types of pectic polysaccharides to be distinguished within compact calli: (i) acidic pectins, probably with few acetyl ester groups, detected without any de-esterification treatment in expanded areas of cell separation but never on middle lamellae between tightly associated cells; (ii) highly methyl-esterified pectins with an expected low acetyl ester content, recognized by the 2F4 antibodies after pectin methylesterase de-esterification, and mostly located on intercellular junctions and on middle lamellae in the central zones of the calli; (iii) highly methyl-esterified and largely acetylated pectins, only localized after alkaline de-esterification, in all primary walls of the compact calli. By contrast, all pectins of friable calli were highly methyland acetyl-esterified. This was consistent with an average degree of methyl-esterification of about 60% measured in both calli, and a higher average degree of acetylation for the friable callus line (85%) compared to the compact one (60%). Accordingly, the pectic fraction (acid-soluble) predominant in both calli was acetyl-esterified to 85% in friable callus and to 22% in compact callus cell walls. Friability of sugar-beet callus is thus correlated with an increase in acetylation of its pectin. Labelings of the Golgi apparatus indicate that the pectic polymers of both callus types are synthesized in dictyosomes in a highly methyl-esterified form and are probably subsequently acetyl-esterified.
Similar content being viewed by others
Abbreviations
- AIR:
-
alcohol-insoluble residue
- DA:
-
degree of acetylation
- DM:
-
degree of methyl-esterification
- MAbs:
-
monoclonal antibodies
- PME:
-
pectin methylesterase
References
Berger, R.K., Reid, P.D. (1979) Role of polygalacturonase in beanleaf abscission. Plant Physiol. 63, 1133–1137
Bertin, C., Rouau, X., Thibault, J.F. (1988) Structure and properties of sugar beet fibres. J. Sci. Food Agric. 44, 15–29
Blaschek, W., Franz, G. (1983) Influence of growth conditions on the composition of cell wall polysaccharides from cultured tobacco cells. Plant Cell Rep. 2, 257–260
Blumenkrantz, N., Asboe-Hansen, G. (1973) New method for quantitative determination of uronic acids. Anal. Biochem. 54, 484–489
Bonfante-Fasolo, P., Vian, B., Perotto, S., Faccio, A., Knox, J.P. (1990) Cellulose and pectin localization in roots of mycorrhizal Allium porrum: labelling between host cell wall and interfacial material. Planta 180, 537–547
De Greef, W., Jacobs, M. (1979) In vitro culture of sugarbeet: description of a cell line with high regeneration capacity. Plant Sci. Lett. 17, 55–61
Delmer, D.P., Stone, B.A. (1988) Biosynthesis of plant cell walls. In: The biochemistry of plants, vol. 14, pp. 373–420, Preiss, J., ed. Academic Press, New York
Englyst, H.N., Cummings, J.H. (1984) Simplified method for the measurement of total non-starch polysaccharides by gas-liquid chromatography of constituent sugars as alditol acetates. Analyst 109, 937–942
Fischer, R.L., Bennett, A.B. (1991) Role of cell wall hydrolases in fruit ripening. Annu. Rev. Plant Physiol. Plant Mol. Biol. 42, 675–703
Fry, S.C. (1988) The growing plant cell wall: chemical and metabolic analysis. Longman, Harlow, UK
Gaspar, Th., Hagège, D., Kevers, C., Penel, C., Crèvecoeur, M., Engelmann, I., Greppin, H., Foidart, J.-M. (1991) When plant teratomas turn into cancers in the absence of pathogens. Physiol. Plant. 83, 696–701
Grant, M.E., Fuller, K.W. (1968) Tissue culture of root cells of Vicia faba. J. Exp. Bot. 19, 667–680
Kevers, C., Coumans, M., De Greef, W., Hofinger, M., Gaspar, T. (1981) Habituation in sugarbeet callus: auxin content, auxin protectors, peroxidase pattern and inhibitors. Physiol. Plant. 51, 281–286
Knox, P.J. (1992a) Cell adhesion, cell separation and plant morphogenesis. Plant J. 2, 137–141
Knox, P.J. (1992b) Molecular probe for the plant cell surface. Protoplasma 167, 1–9
Knox, J.P., Linstead, P.J., King, J., Cooper, C., Roberts, K. (1990) Pectin esterification is spatially regulated both within cell walls and between developing tissues of root apices. Planta 181, 512–521
Kohn, R., Furda, I. (1968) Binding of calcium ions to acetyl derivatives of pectin. Coll. Czech. Chem. Comm. 42, 731–744
Kravtchenko, T.P., Arnould, I., Voragen, A.G.J., Pilnik, W. (1992) Improvement of the selective depolymerization of pectic substances by chemical β-elimination in aqueous solution. Carbohydr. Polym. 19, 237–242
Liners, F., Van Cutsem, P. (1992) Distribution of pectic polysaccharides throughout walls of suspension-cultured carrot cells: an immunocytochemical study. Protoplasma 170, 10–21
Liners, F., Letesson, J.-J., Didembourg, C., Van Cutsem, P. (1989) Monoclonal antibodies against pectin. Recognition of a conformation induced by calcium. Plant Physiol. 91, 1419–1424
Liners, F., Thibault, J.-F., Van Cutsem, P. (1992) Influence of the degree of polymerization of oligogalacturonates and of esterification pattern of pectin on their recognition by monoclonal antibodies. Plant Physiol. 99, 1099–1104
Liu, Q., Berry, A.M. (1991) Localization and characterization of pectic polysaccharides in roots and root nodules of Ceanothus spp. during intercellular infection by Frankia. Protoplasma 163, 93–101
Lynch, M.A., Staehelin, L.A. (1992) Domain-specific and cell type specific localization of two types of cell wall matrix polysaccharides in the clover root tip. J. Cell Biol. 118, 467–479
Matar, D., Catesson, A.M. (1988) Cell plate development and delayed formation of the pectic middle lamella in root meristems. Protoplasma 146, 10–17
Mollard, A., Hustache, G., Barnoud, F. (1973) Les polysaccharides pectiques de la paroi cellulaire dans les tissus de rosier cultivés in vitro. Importance des formes polymères du galactose dans quatre souches de Rosa glauca. Comparaison avec le tissu cambial initial. Physiol. Vég. 11, 539–552
Peretto, R., Perotto, S., Faccio, A., Bonfante-Fasolo, P. (1990) Cell surface in Calluna vulgaris L. hair roots In situ localization of polysaccharidic components. Protoplasma 155, 1–18
Renard, C.M.G.C., Thibault J.F. (1993) Structure and properties of apple and sugar-beet pectins extracted by chelating agents. Carbohydr. Res. 244, 99–114
Rillo, L., Castaldo, D., Giovane, A., Servillo, L., Balestrieri, C., Quagliuolo, L. (1992) Purification and properties of pectin methylesterase from mandarin orange fruit. J. Agric. Food Chem. 40, 591–593
Roland, J.C., Vian, B. (1981) Use of purified endopolygalacturonase for a topochemical study of elongating cell walls at the ultrastructural level. J. Cell Sci. 48, 333–343
Rombouts, F.M., Thibault, J.F. (1986a) Feruloylated pectic substances from sugar-beet pulp. Carbohydr. Res. 154, 177–187
Rombouts, F.M., Thibault, J.F. (1986b) Enzymic and chemical degradation and the fine structure of pectins from sugar-beet pulp. Carbohydr. Res. 154, 189–203
Roy, S., Vian, B., Roland, J.-C. (1992) Immunocytochemical study of the deesterification patterns during cell wall autolysis in the ripening of cherry tomato. Plant Physiol. Biochem. 30, 139–146
Selvendran, R.R. (1985) Developments in the chemistry and biochemistry of pectic and hemicellulosic polymers. J. Cell Sci. Suppl. 2, 51–88
Steel, R.G.D., Torrie, J.H. (1980) Principles and procedures of statistics, a biometrical approach. McGraw-Hill, New York
Thibault, J.F. (1979) Automatisation du dosage des substances pectiques par la méthode au méta-hydroxydiphényl. Lebensm.- Wiss. u.-Technol. 12, 247–251
Thibault, J.F., Rinaudo, M. (1985) Interactions of mono-and divalent counterions with alkalind enzyme-deesterified pectins. Biopolymers 24, 2131–2144
Van Buren, J.P. (1991) Function of pectin in plant tissue structure and firmness. In: The chemistry and technology of pectin (Food science and technology Series), pp. 1–23, Walter, R.H., ed. Academic Press, San Diego
Vandenbosch, K.A., Bradley, D.J., Knox, J.P., Perotto, S.B., Brewin, N.J. (1989) Common components of the infection thread matrix and the intercellular space identified by immunocyto chemical analysis of pea nodules and uninfected roots. EMBO J. 8, 335–342
Vennigerholz, F., Walles, B. (1987) Cytochemical studies of pectin digestion in epidermis with specific cell separation. Protoplasma 140, 110–117
Voragen, A.G.J., Schols, H.A., Pilnik, W. (1986) Determination of the degree of methylation and acetylation of pectins by HPLC. Food Hydrocolloïds 1, 65–70
Vreeland, V., Morse, S.R., Robichaux, R.H., Miller, K.L., Hua, S.T., Laetsch, W.M. (1989) Pectate distribution and esterification in Dubautia leaves and soybean nodules, studied with a fluorescent hybridation probe. Planta 177, 435–446
Wallner, S.J., Nevins, D.J. (1974) Changes in cell walls associated with cell separation in suspension cultures of Paul's scarlet rose. J. Exp. Bot. 25, 1020–1029
Zhang, G.F., Staehelin, L.A. (1992) Functional compartmentation of the Golgi apparatus of plant cells. Immunocytochemical analysis of high-pressure frozennd freeze-substituted sycamore maple suspension culture cells. Plant Physiol. 99, 1070–1083
Author information
Authors and Affiliations
Additional information
Many thanks are due to Mrs. Ch. Devignon (Unité interfacultaire de microscopie électronique, FUNDP, Namur, Belgium) for her technical assistance. F.L. gratefully acknowledges Dr. J.-F. Thibault (Laboratoire de Biochimie et Technologie des glucides, INRA, Nantes, France) for allowing her to stay in his laboratory and Dr. C. Renard for her help with biochemical analyses and her comments on the results. Appreciation is also expressed to P. Vandersmissen (Unité Cell, I.C.P., Bruxelles, Belgium) and to P. Cambier and C. Vinals (FUNDP) for their contribution. The work reported here was supported in part by grants from IRSIA and CGRI, Belgium, to F.L.
Rights and permissions
About this article
Cite this article
Liners, F., Gaspar, T. & Van Cutsem, P. Acetyl- and methyl-esterification of pectins of friable and compact sugar-beet calli: consequences for intercellular adhesion. Planta 192, 545–556 (1994). https://doi.org/10.1007/BF00203593
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00203593