Abstract
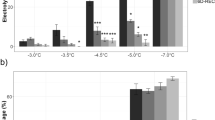
Seasonal alterations in the ultrastructure of the plasma membrane produced by slow freezing were examined in cortical parenchyma cells of mulberry twigs (Morus bombyciz Koidz. cv. Goroji) grown in northern Japan. In freezing-sensitive summer, freezing produced distinct aparticulate domains with accompanying inverted hexagonalII (HII) phase transitions in the plasma membrane. In autumn and spring, during cold acclimation and deacclimation, freezing produced aparticulate domains in the plasma membrane without accompanying Hii phase transitions. In winter, when the twigs were freezing-tolerant, freezing did not produce ultrastructural alterations in the plasma membrane. A significant relationship was recognized between the percentages of cells with aparticulate domains in the plasma membrane, regardless of the presence or absence of HII phase transitions, and the occurrence of freezing injury throughout all seasons and at all freezing temperatures tested in each season. The aparticulate domains in the plasma membranes were shown to be produced by the close apposition of membranes due to freezing-induced dehydration and deformation of cells. Although the precise mechanisms that cause injury as a result of the formation of aparticulate domains in the plasma membrane remain unclear, our results indicate that the development of cold acclimation paralleled the process whereby cells developed the ability to reduce and finally to prevent the formation of aparticulate domains in the plasma membrane that would otherwise result from freezing-induced cellular dehydration and deformation that brings membranes into close proximity with one another.
Similar content being viewed by others
References
Bryant G, Wolf J (1989) Can hydration forces induce lateral phase separations in lamellar phases? Eur J Biophys 16: 369–374
Bryant G, Wolf J (1992) Interfacial forces in cryobiology and anhydrobiology. Cryo-Letters 13: 23–36
Crowe JH, Crowe LM (1984) Effects of dehydration on membranes and membrane stabilization at low water activities. In: Chapman D (ed) Biological membranes, vol 5. Academic Press, London, pp 57–103
Fujikawa S (1987) Mechanical force by growth of extracellular ice crystals is widespread cause for slow freezing injury in tertiary hyphae of mushrooms. Cryo-Letters 8: 156–161
Fujikawa S (1988) Artificial biological membrane ultrastructural changes caused by freezing. Electron Microsc Rev 1: 113–140
Fujikawa S (1990) Cryo-scanning electron microscope and freeze-replica study on the occurrence of slow freezing injury. J Electron Microsc 39: 80–85
Fujikawa S (1991) Freeze facture techniques. In: Harris JR (ed) Electron microscopy in biology: a practical approach. IRL, Oxford, pp 173–201
Fujikawa S, Miura K (1986) Plasma membrane ultrastructural changes caused by mechanical stress in the formation of extracellular ice as a primary cause of slow freezing injury in fruit-bodies of basidiomycetes (Lyophyllum ulmarium Fr. Kühner). Cryobiology 23: 371–382
Fujikawa S, Miura K (1987) Ultrastructural preservation of plasma membranes by non-lethal slow freezing to liquid nitrogen temperature. Cell Struc Func 12: 63–72
Gordon-Kamm WJ, Steponkus PL (1984) Lamellar-to-hexagonalII phase transitions in the plasma membrane of isolated protoplasts after freeze-induced dehydration. Proc Natl Acad Sci USA 81: 6373–6377
Hirsh AG, Williams RJ, Meryman HT (1985) A novel method of natural cryoprotection: intracellular glass formation in deeply frozen Populus. Plant Physiol 79: 41–56
Levitt J (1980) Responses of plants to environmental stresses, vol 1. Academic Press, New York
Lynch DV, Steponkus PL (1987) Plasma membrane lipid alterations associated with cold acclimation of winter rye seedlings (Secale cereale L. cv Puma). Plant Physiol 83: 761–767
Marra J, Israelachvili J (1985) Direct measurements of forces between phosphatidylcholine and phosphatydilethanolamine bilayers in aqueous electrolyte solutions. Biochemistry 24: 4608–4618
Niki T, Sakai A (1981) Ultrastructural changes related to frost hardiness in the cortical parenchyma cells from mulberry twigs. Plant Cell Physiol 22: 171–183
Pearce RS, Willison JHM (1985) A freeze-etch study of the effects of extracellular freezing on cellular membranes of wheat. Planta 163: 304–316
Pihakaski K, Steponkus PL (1987) Freeze-induced phase transitions in the plasma membrane of isolated protoplasts. Physiol Plantarum 69: 666–674
Rand RP (1981) Interacting phospholipid bilayers: measured forces and induced structural changes. Annu Rev Biophys Bioeng 10: 277–314
Rand RP, Parsegian VA (1989) Hydration forces between phospholipid bilayers. Biochim Biophys Acta 988: 351–376
Sakai A (1960) Survival of the twigs of woody plants at -196°C. Nature 185: 393–394
Sakai A, Yoshida S (1968) The role of sugar and related compounds in variations of freezing resistance. Cryobiology 5: 160–174
Siminovitch D, Rheaume B, Pomeroy K, Lepage M (1968) Phospholipid, protein, and nucleic acid increases in protoplasm and membrane structures associated with development of extreme freezing resistance in black locust tree cells. Cryobiology 5: 202–225
Steponkus PL (1984) Role of the plasma membrane in freezing injury and cold acclimation. Annu Rev Plant Physiol 35: 543–584
Steponkus PL, Lynch DV (1989) Freeze/thaw-induced destabilization of the plasma membrane and the effects of cold acclimation. J Bioenerg 21: 21–41
Steponkus PL, Webb MS (1992) Freeze-induced dehydration and membrane destabilization in plants. In: Somero GN, Osmond CB and Bolis DH (eds) Water and life: comparative analysis of water relationship at the organismic, cellular and molecular level. Springer, Berlin Heidelberg New York, pp 338–362
Webb MS, Steponkus PL (1993) Freeze-induced membrane ultrastructural alterations in rye (Secale cereals) leaves. Plant Physiol 101: 955–963
Yoshida S (1984) Chemical and biophysical changes in the plasma membrane during cold acclimation of mulberry bark cells (Morus bombycis Koids. cv Goroji). Plant Physiol 76: 257–265
Yoshida S, Uemura M (1984) Protein and lipid compositions of isolated plasma membranes from orchard grass (Dactylis glomerata L.) and changes during cold acclimation. Plant Physiol 75: 31–37
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fujikawa, S. Seasonal ultrastructural alterations in the plasma membrane produced by slow freezing in cortical tissues of mulberry (Morus bombyciz Koidz. cv. Goroji). Trees 8, 288–296 (1994). https://doi.org/10.1007/BF00202673
Issue Date:
DOI: https://doi.org/10.1007/BF00202673