Abstract
The interaction of antiparasitic drugs with the polymorphic cytochrome P450 2D6 was studied in human liver microsomes.
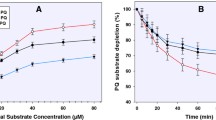
Of ten different drugs tested, three quinolines, oxamniquine, primaquine and chloroquine inhibited microsomal CYP2D6-catalysed formation of 1′hydroxybufuralol at concentrations that might have clinical consequences in drug use. These drugs inhibited competitively bufuralol metabolism with K i values of 22, 23 and 15 μM, respectively, indicative of high affinity for the CYP2D6-active site. The results imply that oxamniquine, primaquine and chloroquine could be substrates of cytochrome P4502 D6 or that they are potent non-substrate inhibitors of the enzyme similar to quinidine.
In either case, the inhibition of CYP2D6 by these agents could lead to interference with in vivo population-phenotyping procedures in the tropical regions where treatment with the drugs is common.
Similar content being viewed by others
References
Eichelbaum M, Gross AS (1990) The genetic polymorphism of debrisoquine/sparteine metabolism — clinical aspects. Pharmacol Ther 46: 377–394
Zanger UM, Vilbois F, Hardwick JP, Meyer UA (1988) Absence of hepatic cytochrome P450 bufI causes genetically deficient debrisoquine oxidation in man. Biochemistry 27: 5447–5454
Meyer UA, Skoda RC, Zanger UM, Heim MH, Broly F (1992) The genetic polymorphism of debrisoquine/sparteine metabolism — molecular mechanisms. In: Kalow W (ed) Pharmacogenetics of drug metabolism. Pergamon Press, New York, pp 609–623
Daly AK, Cholerton S, Gregory W, Idle WR (1993) Metabolic polymorphisms. Pharmacol Ther 57: 129–160
Koymans L, Vermeulen NPE, Acker SABE, Koppele JM, Heykants JP, Lavrijsen K, Meuldermans W, Kelder GMD (1992) A predictive model for substrates of cytochrome P450-debrisoquine (2D6). Chem Res Toxicol 5: 211–219
Fonner-Pfister R, Meyer UA (1988) Xenobiotic and endobiotic inhibitors of cytochrome P450 db1 function. The target of the debrisoquine/sparteine type polymorphism. Biochem Pharmacol 37: 3829–3835
Strobl GR, Kruedener S, Stockigt J, Guengerich FP, Wolff T (1993) Development of a pharmacophore for inhibition of human liver cytochrome P450 2D6: molecular modeling and inhibition studies. J Med Chem 36: 1136–1145
Manson-Bahr PEC, Bell DR (1987) Manson's tropical diseases, 19th edn. Bailliere Tindall, London
Gustafsson LL, Beerman B, Abdi YA (1987) Handbook of drugs for tropical parasitic infections. Taylor & Francis, Philadelphia
James MD, Gilles A (1985) Human antiparasitic drug pharmacology and usage. Wiley, Chichester
Bahr C von, Groth C, Jansson H, Lundgren G, Lind M, Glaumann H (1980) Drug metabolism in human liver in vitro: establishment of a human liver bank. Clin Pharmacol Ther 24: 711–725
Kronbach T, Mathys D, Gut J, Catin T, Meyer UA (1987) High-performance liquid chromatography assays for bufuralol 1′hydroxylase, debrisoquine 4-hydroxylase, and dextromethorphan O-demethylase in microsomes and purified cytochrome P450 isozymes of human liver. Anal Biochem 162: 24–32
Lancaster DL, Adio RA, Tai KK, Simooya OO, Broadbead GD, Tucker GT, Lennard MS (1990) Inhibition of metoprolol metabolism by chloroquine and other antimalarial drugs. J Pharm Pharmacol 42: 267–271
Kokwaro GO, Taylor G (1991) Oxamniquine pharmacokinetics in healthy Kenyan African volunteers. East Afr Med J 68: 359–363
Mackenzie AH (1983) Pharmacologic actions of 4-aminoquinoline compounds. Am J Med 18: 5–9
Ette EI, Essien EE, Thomas WOA, Brown-Awala EA (1989) Pharmacokinetics of chloroquine and some of its metabolites in healthy volunteers: a single dose study. J Clin Pharmacol 29: 457–462
Wolff T, Distlerath LM, Worthington MT, Groopman JD, Hammons GJ, Kadlubar FF, Prough RA, Martin MV, Guengerich FP (1985) Substrate specificity of human liver cytochrome P450 debrisoquine 4-hydroxylase probed using immunochemical inhibition and chemical modeling. Cancer Res 45: 2116–2122
Murray M (1987) Mechanisms of the inhibition of cytochrome P450-mediated drug oxidation by therapeutic agents. Drug Metab Rev 48: 55–81
Koymans L, Kelder GMD, Koppele JM, Vermeulen NPE (1993) Cytochromes P450: their active-site structure and mechanism of oxidation. Drug Metab Rev 25: 325–387
Mikus G, Ha AR, Vozeh S, Zekorn C, Follath F, Eichelbaum M (1986) Pharmcokinetics and metabolism of quinidine in extensive and poor metabolisers of sparteine. Eur J Clin Pharmacol 31: 69–72
White NJ (1985) Clinical pharmacokinetics of antimalarial drugs. Clin Pharmacokinet 10: 187–215
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hasler, J.A., Johansson, I. & Masimirembwa, C.M. Inhibitory effects of antiparasitic drugs on cytochrome P450 2D6. Eur J Clin Pharmacol 48, 35–38 (1995). https://doi.org/10.1007/BF00202169
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00202169