Abstract
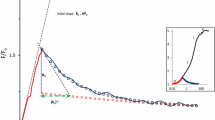
The dark-relaxation kinetics of variable fluorescence, Fv, in intact green leaves of Pisum stativum L. and Dolichos lablab L. were analyzed using modulated fluorometers. Fast (t1/2 = 1 s) and slow (t1/2 = 7–8 s) phases in fv dark-decay kinetics were observed; the rate and the relative contribution of each phase in total relaxation depended upon the fluence rate of the actinic light and the point in the induction curve at which the actinic light was switched off. The rate of the slow phase was accelerated markedly by illumination with far-red light; the slow phase was abolished by methyl viologen. The halftime of the fast phase of Fv dark decay decreased from 250 ms in dark-adapted leaves to 12–15 ms upon adaptation to red light which is absorbed by PSII. The analysis of the effect of far-red light, which is absorbed mainly by PSI, on Fv dark decay indicates that the slow phase develops when a fraction of QA − (the primary stable electron acceptor of PSII) cannot transfer electrons to PSI because of limitation on the availability of P700+ (the primary electron donor of PSI). After prolonged illumination of dark-adapted leaves in red (PSII-absorbed) light, a transient. Fv rise appears which is prevented by far-red (PSI-absorbed) light. This transient fv rise reflects the accumulation of QA − in the dark. The observation of this transient Fv rise even in the presence of the uncoupler carbonylcyanide m-chlorophenyl hydrazone (CCCP) indicates that a mechanism other than ATP-driven back-transfer of electrons to QA may be responsible for the phenomenon. It is suggested that the fast phase in Fv dark-decay kinetics represents the reoxidation of QA − by the electron-transport chain to PSI, whereas the slow phase is likely to be related to the interaction of QA − with the donor side of PSII.
Similar content being viewed by others
Abbreviations
- CCCP:
-
carbonylcyanide m-chlorophenylhydrazone
- FO :
-
initial fluorescence level
- Fv :
-
variable fluorescence
- P700:
-
primary electron donor of PSI
- PSI, II:
-
photosystem I, II
- QA (QA −) QB (QB −):
-
primary and secondary stable electron acceptor of PSII in oxidized (reduced) state
References
Bennoun, P. (1970) Reoxydation du quencher de fluorescence “Q” en presence de 3-(3,4-dichlorophenyl)-1.1-dimethyluree. Bioch im. Biophys. Acta 216, 357–363
Bonaventura, C., Myers, J. (1969) Fluorescence and oxygen evolution from Chlorella pyrenoidosa. Biochim. Biophys. Acta 189, 366–383
Bradbury, M., Baker, N.R. (1984) A quantitative determination of photochemical and non-photochemical quenching during the slow phase of the chlorophyll fluorescence induction curve of bean leaves. Biochim. Biophys. Acta 765, 275–290
Buchanan, R. (1980) Role of light regulators of chloroplast enzymes. Annu. Rev. Plant Physiol. 31, 341–356
Bukhov, N.G., Mohanty, P., Rakhimberdieva, M.G., Karapetyan, N.V. (1991) Analysis of fluorescence induction transients: major part of light-induced decline in variable fluorescence may not be linked to ApH accumulation. Photosynthetica 25, 86–94
Cao, J., Govindjee (1990) Chlorophyll a fluorescence transient as an indicator of active and inactive photosystem II in thylakoid membranes. Biochim. Biophys. Acta 1015, 180–188
Chylla, R.A., Whitmarsh, J. (1989) Inactive photosystem II complexes in leaves. Turnover rate and quantitation. Plant Physiol. 80, 765–772
Chylla, R.A., Garab, G., Whitmarsh, J. (1987) Evidence for slow turnover in a fraction of photosystem II complexes in thylakoid membranes. Biochim. Biophys. Acta 824, 562–571
Duysens, L.N.M., Sweers, H.E. (1963) Mechanism of two photochemical reactions in algae as studied by means of fluorescence. In: Studies on microalgae and photosynthetic bacteria, pp. 353–372, Miyachi, S., ed. University of Tokyo Press, Tokyo
Forbush, B., Kok, B. (1968) Reactions between primary and secondary electron acceptors of photosystem II of photosynthesis. Biochim. Biophys. Acta 162, 243–253
Govindjee, Papageorgiou, G. (1971) Chlorophyll fluorescence and photosynthesis: fluorescence transients. In: Photophysiology, vol. 6, pp. 1–49, Giese, A., ed. Academic Press, New York
Haehnel, W. (1984) Photosynthetic electron transport in higher plants. Annu. Rev. Plant Physiol. 35, 659–693
Heath, R.L. (1970) Kinetic studies on the fluorescence quenching in isolated chloroplasts. Biophys. J. 10, 1173–1180
Homann, P.H. (1971) Action of carbonylcyanide m-chlorophenylhydrazone on electron transport and fluorescence of isolated chloroplasts. Biochim. Biophys. Acta 245, 129–143
Joliot, P., Joliot, A., Bouges, B., Barbieri, G. (1971) Studies on photosystem II centers by comparative measurements of lumiescence, fluorescence and oxygen evolution. Photochem. Photobiol. 14, 287–305
Karapetyan, N.V., Klimov, V.V. (1971) A device for measurements of the kinetics of light-induced fluorescence changes of photosynthetic organisms. (In russ.) Fiziol. Rast. 18, 223–228
Karapetyan, N.V., Klimov, V.V., Krasnovsky, A.A. (1973) Light-induced changes in the fluorescence yield of particles obtained by digitonin fragmentation of chloroplasts. Photosynthetica 7, 330–337
Kautsky, H., Hirsch, A. (1931) Neue Versuche zur Kohlen-säureassimilation. Naturwissenschaften 19, 694–699
Laisk, A., Oja, V., Kiirats, O., Raschke, K., Heber, U. (1989) The state of photosynthetic apparatus in leaves as analyzed by rapid gas exchange and optical methods: the pH of the chloroplast stroma and activation of enzymes in vivo. Planta 177, 350–358
Lavergne, G. (1974) Fluorescence induction in algae and chloroplasts. Photochem. Photobiol. 20, 377–386
Malkin, S. (1977) Delayed luminescence. In: Primary processes of photosynthesis, pp. 351–431. Barber, J., ed. Elsevier, The Hague, The Netherlands
Melandri, B.A. (1977) The high energy state. In: Encyclopedia of plant physiology, N.S., vol. 5: Photosynthesis I, pp. 358–368, Trebst, A., Avron, M., eds. Springer-Verlag, New York
Mohanty, P., Mar, T., Govindjee (1971) Action of hydroxylamine on red algae Porphyridium cruentum. Biochim. Biophys. Acta 253, 213–221
Munday, J.C., Jr., Govindjee (1969) Light-induced changes in the fluorescence yield of chlorophyll a in vivo. III. The dip and the peak in the fluorescence transients of Chlorella pyrenoidosa. Biophys. J. 9, 1–19
Renger, G. (1972) The action of 2-anilinothiophenes as accelerators of the deactivation reactions in the watersplitting enzyme system of photosynthesis. Biochim. Biophys. Acta 256, 428–439
Robinson, H.M., Crofts, A.R. (1983) Kinetics of the oxidationreduction reactions of the photosystem II quinone acceptor complex and the pathway for deactivation. FEBS Lett. 153, 221–226
Satoh, K. (1980) Mechanisms of photoactivation of electron transport in intact Bryopsis chloroplasts. Plant Physiol. 70, 1413–1416
Schreiber, U. (1986) Detection of rapid induction kinetics with a new type of high-frequency modulated chlorophyll fluorometer. Photosynth. Res. 9, 261–272
Schreiber, U., Avron, M. (1979) Properties of ATP-driven revers ible electron flow in chloroplasts. Biochim. Biophys. Acta 546, 436–447
Walker, D.A. (1981) Secondary fluorescence kinetics of spinach leaves in relation to the onset of photosynthetic carbon assimilation. Planta 153, 273–282
Author information
Authors and Affiliations
Additional information
Supported by grant B6.1/88 DST, Govt. of India.
Rights and permissions
About this article
Cite this article
Bukhov, N.G., Mohanty, P., Rakhimberdieva, M.G. et al. Analysis of dark-relaxation kinetics of variable fluorescence in intact leaves. Planta 187, 122–127 (1992). https://doi.org/10.1007/BF00201633
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00201633