Abstract
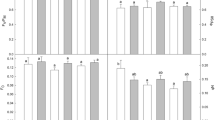
The properties of two Calvin-cycle key enzymes, i.e. stromal fructose-1,6-bisphosphatase (sFBPase) and ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) were studied in the cultivated tomato (Lycopersicon esculentum Mill.) and in four lines of a wild tomato (L. peruvianum Mill.) from different altitudes. During chilling for 14 d at 10°C and low light, the activation energy (EA) of the reaction catalyzed by sFBPase decreased by 5–10 kJ·mol−1 in L. esculentum and the three L. peruvianum lines from high altitudes. In L. peruvianum, no loss or only small losses of enzyme activity were observed during the chilling. Together with the change in EA, this indicates that the latter species is able to acclimate its Calvin-cycle enzymes to low temperatures. In L. esculentum, the chilling stress resulted in the irreversible loss of 57% of the initial sFBPase activity. Under moderately photoinhibiting chilling conditions for 3 d, the L. peruvianum line from an intermediate altitude showed the largest decreases in both the ratio of variable to maximum chlorophyll fluorescence (Fv/Fm) and the in-vivo activation state of sFBPase, while the other L. peruvianum lines showed no inhibition of sFBPase activation. Ribulose-1,5-bisphosphate carboxylase/oxygenase was isolated by differential ammonium-sulfate precipitation and gel filtration and characterized by two-dimensional electrophoresis. The enzyme from L. esculentum had three isoforms of the small subunit of Rubisco, each with different isoelectric points. Of these, the L. peruvianum enzyme contained only the two more-acidic isoforms. Arrhenius plots of the specific activity of purified Rubisco showed breakpoints at approx. 17°C. Upon chilling, the specific activity of the enzyme from L. esculentum decreased by 51%, while EA below the breakpoint temperature increased from 129 to 189 kJ·mol−1. In contrast, Rubisco from the L. peruvianum lines from high altitudes was unaffected by chilling. We tested several possibile explanations for Rubisco inactivation, using two-dimensional electrophoresis, analytical ultracentrifugation, gel filtration and inhibitor tests. No indications were found for differential expression of the subunit isoforms, proteolysis, aggregation, subunit disassembly, or inhibitor accumulation in the enzyme from chilled L. esculentum. We suggest that the activity loss in the L. esculentum enzyme upon chilling is the result of a modification of sulfhydryl groups or other sidechains of the protein.
Similar content being viewed by others
Abbreviations
- a.s.l.:
-
above sea level
- Chl:
-
chlorophyll
- DTT:
-
dithiothreitol
- EA :
-
activation energy
- FBP:
-
fructose-1,6-bisphosphate
- Fv/Fm :
-
ratio of variable to maximum chlorophyll fluorescence
- HL:
-
high light (500 μmol photons·m−2·s−1)
- LSU:
-
large subunit of Rubisco
- ME:
-
2-mercaptoethanol
- Rubisco:
-
ribulose-1,5-bisphosphate carboxylase/oxygenase
- RuBP:
-
ribulose-1,5-bisphosphate
- sFBPase:
-
stromal fructose-1,6-bisphosphatase
- SSU:
-
small subunit of Rubisco
References
Badger, M.R., Björkman, O., Armond, P.A. (1982) Analysis of photosynthetic response and adaptation to temperature in higher plants: Temperature acclimation in the desert evergreen Nerium oleander L. Plant Cell Environ. 5, 85–99
Bradshaw, A.D. (1965) Evolutionary significance of phenotypic plasticity in plants. Adv. Genet. 13, 115–155
Brüggemann, W., Dauborn, B. (1993) Long-term chilling of young tomato plants under low light. III. Leaf development as reflected by photosynthesis parameters. Plant Cell Physiol. 34, 1251–1257
Brüggemann, W., Linger, P. (1994) Long-term chilling of young tomato plants under low light. IV. Differential responses of chlorophyll fluorescence quenching coefficients in Lycopersicon species of different chilling sensitivity. Plant Cell Physiol., in press
Brüggemann, W., van der Kooij, T.A.W., van Hasselt, P.R. (1992a) Long-term chilling of young tomato plants under low light and subsequent recovery. I. Growth, development and photosynthesis. Planta 186, 172–178
Brüggemann, W., van der Kooij, T.A.W., van Hasselt, P.R. (1992b) Long-term chilling of young tomato plants under low light and subsequent recovery. II. Chlorophyll fluorescence, carbon metabolism and activity of ribulose-1,5-bisphosphate carboxylase/oxygenase. Planta 186, 179–187
Chabot, B.F., Chabot, J.F., Billings, W.D. (1972) Ribulose-1,5-diphosphate carboxylase activity in arctic and alpine populations of Oxyria digyna. Photosynthetica 6, 364–369
Chen, K., Kung, S.D., Gray, J.C., Wildman, S.G. (1976) Subunit polypeptide composition of fraction I protein from various plant species. Plant Sci. Lett. 7, 429–434
Flachmann, R., Bohnert, H.J. (1992) Replacement of a conserved arginine in the assembly domaine of ribulose-1,5-bisphosphate carboxylase/oxygenase small subunit interferes with holoenzyme formation. J. Biol. Chem. 267, 10576–10582
Gerhardt, R., Stitt, M., Heldt, H.W. (1987) Subcellular metabolite levels in spinach leaves. Regulation of sucrose synthesis during diurnal alterations in photosynthetic partitioning. Plant Physiol. 83, 399–407
Grafflage, S., Krause, G.H. (1993) Alterations of properties of ribulose bisphosphate carboxylase related to cold acclimation. In: Advances in cold hardiness, pp. 113–124, Lee, P.L., Christersson, L., eds. CRC Press, Boca Raton
Hall, N.P., McCurry, S.D., Tolbert, N.E. (1981) Storage and maintaining activity of ribulose bisphosphate carboxylase/oxygenase. Plant Physiol. 67, 1220–1223
Holaday, A.S., Martindale, W., Alred, R., Brooks, A.L., Leegood, R.C. (1992) Changes in activities of enzymes of carbon metabolism in leaves during exposure of plants to low temperature. Plant Physiol. 98, 1105–1114
Huner, N.P.A., Carter, J.V. (1982) Differential subunit aggregation of a purified protein from cold-hardened and unhardened Puma rye. Z. Pflanzenphysiol. 106, 179–184
Huner, N.P.A., Macdowall, F.D.H. (1978) Evidence for an in vivo conformational change in ribulose bisphosphate carboxylase-oxygenase from Puma rye during cold adaptation. Can. J. Biochem. 56, 1154–1161
Huner, N.P.A., Palta, J.P., Li, P.H., Carter, J.V. (1981) Comparison of the structure and function of ribulose bisphosphate carboxylase-oxygenase from a cold-hardy and nonhardy potato species. Can. J. Biochem. 59, 280–289
Kawashima, N., Wildman, S.G. (1970) Fraction I protein. Annu. Rev. Plant Physiol. 21, 325–358
Krause, G.H., Weis, E. (1991) Chlorophyll fluorescence and photosynthesis: The basics. Annu. Rev. Plant Physiol. Plant Mol. Biol. 42, 313–349
Levitt, J. (1980) Responses of plants to environmental stress, Vol. I., Chilling, freezing and high temperature stress. Academic Press, New York
Lilley, R. McC., Walker, D.A. (1974) An improved spectrophotometric assay for ribulose bisphosphate carboxylase. Biochim. Biophys. Acta 358, 226–229
McIntosh, L., Poulsen, C., Bogorad, L. (1980) Chloroplast gene sequence for the large subunit of ribulose bisphosphate carboxylase of maize. Nature 288, 556–560
Miyano, M. (1983) A sharp trasition in activity and conformation of tobacco ribulose-1,5-bisphosphate carboxylase/oxygenase. Biochim. Biophys. Acta 743, 207–211
Moore, B.D., Seeman, J.R. (1991) Leaf metabolism of 2′-carboxyarabinitol. (Abstr.) Plant Physiol. 96, Suppl 49
O'Farrell, P.H. (1975) High resolution two-dimensional electrophoresis of proteins. J. Biol. Chem. 250, 4007–4021
Paulsen, J.M., Lane, M.D. (1966) Spinach ribulose diphosphate carboxylase. I. Purification and properties of the enzyme. Biochemistry 5, 2350–2357
Pichersky, E., Bernatzky, R., Tanksley, S.D., Cashmore, A.R. (1986) Evidence for selection as a mechanism in the concerted evolution of Lycopersicon esculentum (tomato) genes encoding the small subunit of ribulose-1,5-bisphosphate carboxylase-oxygenase. Proc. Natl. Acad. Sci. USA 83, 3880–3884
Salvucci, M.E. (1989) Regulation of rubisco activity in vivo. Physiol. Plant. 77, 164–171
Sassenrath, G.F., Ort, D.R. (1990) The relationship between inhibition of photosynthesis at low temperature and the inhibition of photosynthesis after rewarming in chill-sensitive tomato. Plant Physiol. Biochem. 28, 457–465
Sassenrath, G.F., Ort, D.R., Portis, A.R. (1987) Effect of chilling on the activity of enzymes of the photosynthetic carbon reduction cycle. Progr. Photosynth. Res. IV, 103–106
Sassenrath, G.F., Ort, D.R., Portis, A.R. (1990) Impaired reductive activation of stromal bisphosphatases in tomato leaves following low-temperature exposure at high light. Arch. Biochem. Biophys. 282, 302–308
Servaites, J.C. (1985) Binding of a phosphorylated inhibtor to ribulose bisphosphate carboxylase/oxygenase during the night. Plant Physiol. 78, 839–843
Shinozaki, K., Suguira, M. (1982) The nucleotide sequence of the tobacco chloroplast gene for the large subunit of ribulose-1,5-bisphosphate carboxylase-oxygenase. Gene 20, 91–102
von Caemmerer, S., Farquhar, S.D. (1981) Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153, 376–387
Wang, C.Y. (ed.) (1990) Chilling injury in horticultural crops. CRC Press, Boca Raton
Wildman, S.G. (1979) Aspects of fraction 1 protein evolution. Arch. Biochem. Biophys. 196, 598–610
Winter, P., Herrmann, R.G. (1987) A five-base-pair deletion in the gene for the large subunit causes the lesion in the ribulose bisphosphate carboxylase/oxygenase-deficient mutant sigma of Oenothera hookeri. Bot. Acta 101, 42–48
Wishnick, M., Lane, M.D. (1971) Ribulose diphosphate carboxylase from spinach leaves. Methods Enzymol. 23, 570–577
Zurawski, G., Perrot, B., Bottomley, W, Whitfield, P. (1981) The structure of the gene for the large subunit of ribulose-1,5-bisphosphate carboxylase from spinach chloroplast DNA. Nucleic Acid Res. 9, 3251–3270
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Brüggemann, W., Klaucke, S. & Maas-Kantel, K. Long-term chilling of young tomato plants under low light. Planta 194, 160–168 (1994). https://doi.org/10.1007/BF00196384
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00196384