Abstract
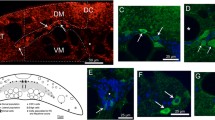
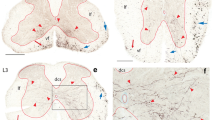
Studies on the effect of axotomy on adult intrinsic central projection neurons have generally assumed that the severed proximal axonal stumps were still capable of retrogradely transporting tracer at varying times after injury. Failure of transport was interpreted as neuronal death, which is at odds with current understanding that central projection neurons survive distal axotomy. We used lumbar spinal cord-projecting rubrospinal neurons of the rat as a model to evaluate the ability of injured neurons to transport tracer retrogradely at different times after distal axotomy. We examined only the caudal part of the red nucleus, since rubrospinal neurons are concentrated here. In control animals, tracer applied to the rubrospinal tract at the T10 vertebral level labeled ventrolateral rubral neurons, while C3 application marked all rubral neurons. From 3 days after a T10 axotomy and tracer application, most ventrolateral neurons were no longer labeled by another tracer application at the C3 vertebral level via an axonal cut. The phenomenon was not caused by tracer toxicity, since a T10 tractotomy without tracer application also prevented these axotomized neurons from being labeled when treated similarly. Thus, neuronal retrograde transport capability was seriously retarded 3 days after a distal axotomy. Loss of retrograde transport may merely suggest that a mechanism no longer in service has been switched off, or perhaps it may insulate injured neurons from the effect of lesion site-derived factors. Using this property, we were able to localize cervical spinal cord-projecting rubrospinal neurons in the caudal red nucleus. Results show that although they concentrate in the dorsomedial region, some neurons were found to extend into the ventrolateral part of the nucleus.
Similar content being viewed by others
References
Antal M, Sholomenko GN, Moschovakis AK, Storm-Mathisen J, Heizmann CW, Hunziker W (1992) The termination pattern and postsynaptic targets of rubrospinal fibers in the rat spinal cord: a light and electron microscopic study. J Comp Neurol 325:22–37
Barron KD, Dentinger MP (1978) Abnormal ultrastructural appearances in axons of feline pericruciate cortex after lateral funiculotomy. Acta Neuropathol (Berl) 44:1–8
Barron KD, Dentinger MP (1979) Cytologic observations on axotomized feline Betz cells. I. Qualitative electron microscopic findings. J Neuropathol Exp Neurol 38:128–151
Egan DA, Flumerfelt BA, Gwyn DG (1977) A light and electron microscopic study of axon reaction in the red nucleus of the rat following cervical and thoracic lesions. Neuropathol Appl Neurobiol 3:423–439
Feringa ER, Vahsing HL, Smith BE (1983) Retrograde transport in corticospinal neurons after spinal cord transection. Neurology 33:478–482
Feringa ER, Wayne JG, Vahlsing HL (1984) Histologic evidence for death of cortical neurons after spinal cord transection. Neurology 34:1002–1006
Frizell M, McLean WG, Sjostrand J (1976) Retrograde axonal transport of rapidly migrating labelled proteins and glycoproteins in regenerating peripheral nerves. J Neurochem 27:191–196
Goshgarian HG, Koistinen JM, Schmidt ER (1983) Cell death and changes in the retrograde transport of horseradish peroxidase in rubrospinal neurons following spinal cord hemisection in the adult rat. J Comp Neurol 214:251–257
Halperin JJ, La Vail JH (1975) A study of the dynamics of retrograde transport and accumulation of horseradish peroxidase in injured neurons. Brain Res 100:253–269
Huisman AM, Kuypers HGJM, Verburgh CA (1981) Quantitative differences in collaterization of the descending spinal pathways from red nucleus and other brain stem cell groups in rat as demonstrated with the multiple fluorescent retrograde tracer technique. Brain Res 209:271–286
Kalil K, Schneider GE (1975) Retrograde cortical and axonal changes following lesions of the pyramidal tract. Brain Res 89:15–27
Kristensson K, Sjostrand J (1972) Retrograde transport of protein tracer in the rabbit hypoglossal nerve during regeneration. Brain Res 45:175–181
Lakke EAJF, Marani E (1991) Prenatal descent of rubrospinal fibers through the spinal cord of the rat. J Comp Neurol 314:67–78
Lassek AM (1942) The pyramidal tract: a study of retrograde degeneration in the monkey. Arch Neurol Psych 48:561–567
McBride RL, Feringa ER, Garver MK, Williams JK (1989) Prelabeled red nucleus and sensorimotor cortex neurons of the rat survive 10 and 20 weeks after spinal cord transection. J Neuropathol Exp Neurol 48:568–576
Merline M, Kalil K (1990) Cell death of corticospinal neurons is induced by axotomy before but not after innervation of spinal targets. J Comp Neurol 296:506–516
Murray HM, Gurule K (1979) Origin of the rubrospinal tract of the rat. Neurosci Lett 14:19–23
Olsson TP, Forsberg I, Kristensson K (1978) Uptake and retrograde transport of horseradish peroxidase in regenerating facial motor neurons of the mouse. J Neurocytol 7:323–336
Pernet U, Hepp-Reymond M-C (1975) Retrograde Degeneration der Pyramidenbahnzellen im motorischen Kortex beim Affen (Macaca fascicularis). Acta Anat (Basel) 92:552–561
Prendergast J, Stelzner DJ (1976) Changes in the magnocellular portion of the red nucleus following thoracic transection in the neonatal and adult rat. J Comp Neurol 166:163–172
Richardson PM, Issa VMK, Aguayo AJ (1984) Regeneration of long spinal axons in the rat. J Neurocytol 13:165–182
Shieh JY, Leong SK, Wong WC (1983) Origin of the rubrospinal tract in neonatal, developing, and mature rats. J Comp Neurol 214:79–86
Tetzlaff W, Alexander SW, Miller FD, Bisby MA (1991) Responses of facial and rubrospinal neurons to axotomy: changes in mRNA expression for cytoskeletal proteins and GAP-43. J Neurosci 11:2528–2544
Tseng G-F, Prince DA (1990) Neuronal properties of identified corticospinal cells in vitro following spinal axotomy in vivo. Soc Neurosci Abstr 16:1163
Tseng G-F, Prince DA (1991) Morphological characteristics of double-labelled axotomized corticospinal neurons studied in vitro. Soc Neurosci Abstr 17:311
Vallee RB, Bloom GS (1991) Mechanisms of fast and slow axonal transport. Annu Rev Neurosci 14:59–92
Vallee RB, Shpetner MP (1990) Motor proteins of cytoplasmic microtubules. Annu Rev Biochem 59:909–932
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tseng, GF., Shu, J., Huang, SJ. et al. A time-dependent loss of retrograde transport ability in distally axotomized rubrospinal neurons. Anat Embryol 191, 243–249 (1995). https://doi.org/10.1007/BF00187823
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00187823