Abstract
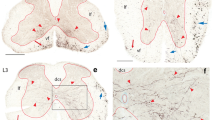
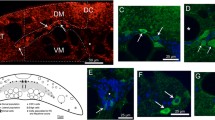
The effect of axotomy at cervical and lumbar spinal levels upon the ability of rubrospinal neurons to retrogradely transport tracer was compared. Unilateral rubrospinal tractotomy was performed first at C5 and, after a few days, at C2 vertebral levels. Different retrograde tracers were applied at the lesioned sites right after tractotomy. Tracer applied at C5 labeled both cervical and lumbar-cord-projecting neurons. Tracer applied at C2 also labeled both groups of neurons if performed 2 days after that at C5; however, only cervical-cord-projecting neurons were labeled when it was performed 3 or 5 days after that at C5. In another set of experiments, a T10 tractotomy without tracer application was performed 2 or 5 days prior to the C5/C2 series of tract lesions. When preceded by a T10 lesion 2 days in advance, tracer applied at C5 labeled both cervical and lumbar-cord-projecting neurons. However, a T10 lesion 5 days in advance resulted in the labeling of only cervical-cord-projecting neurons by the tracer applied at C5. In either case, tracer applied at C2 consistently labeled only cervical-cord-projecting neurons, irrespective of the intervals — 2, 3, or 5 days — allowed between C5 and C2 lesions. Most neurons labeled from C2 were also double-labeled by the tracer applied at C5. Thus, unlike lumbar-cord-projecting counterparts, cervical-cord-projecting rubrospinal neurons retain the ability to uptake and/or transport retrograde tracer several days following axotomy. This implies that cervical-cord-projecting rubrospinal neurons survive in a different functional state from their lumbar-cord-projecting counterparts following axonal injury.
Similar content being viewed by others
References
Feringa ER, Vahlsing HL, Smith BE (1983) Retrograde transport in corticospinal neurons after spinal cord transection. Neurology 33:478–482
Feringa ER, Wayne JG, Vahlsing HL (1984) Histologic evidence for death of cortical neurons after spinal cord transection. Neurology 34:1002–1006
Frizell M, McLean WG, Sjostrand J (1976) Retrograde axonal transport of rapidly migrating labelled proteins and glycoproteins in regenerating peripheral nerves. J Neurochem 27:191–196
Gallant PE, Hammar K, Reese TS (1995) Cytoplasmic constriction and vesiculation after axotomy in squid giant axon. J Neurocytol 24:943–954
Goshgarian HG, Koistinen JM, Schmidt ER (1983) Cell death and changes in the retrograde transport of horseradish peroxidase in rubrospinal neurons following spinal cord hemisection in the adult rat. J Comp Neurol 214:251–257
Halperin JJ, LaVail JH (1975) A study of the dynamics of retrograde transport and accumulation of horseradish peroxidase in injured neurons. Brain Res 100:253–269
Huisman AM, Kuypers HGJM, Verburgh CA (1981) Quantitative differences in collaterization of the descending spinal pathways from red nucleus and other brain stem cell groups in rat as demonstrated with the multiple fluorescent retrograde tracer-technique. Brain Res 209:271–286
Kristensson K, Sjostrand J (1972) Retrograde transport of protein tracer in the rabbit hypoglossal nerve during regeneration. Brain Res 45:175–181
McBride RL, Feringa ER, Garver MK, Williams JK (1989) Prelabeled red nucleus and sensorimotor cortex neurons of the rat survive 10 and 20 weeks after spinal cord transection. J Neuropathol Exp Neurol 48:568–576
Merline M, Kalil K (1990) Cell death of corticospinal neurons is induced by axotomy before but not after innervation of spinal targets. J Comp Neurol 296:506–516
Murray HM, Gurule K (1979) Origin of the rubrospinal tract of the rat. Neurosci Lett 14:19–23
Olsson TP, Forsberg I, Kristensson K (1978) Uptake and retrograde transport of horseradish peroxidase in regenerating facial motor neurons of the mouse. J Neurocytol 7:323–336
Parton RG, Simons K, Dotti CG (1992) Axonal and dendritic endocytic pathways in cultured neurons. J Cell Biol 119:123–137
Richardson PM, Issa VMK, Aguayo AJ (1984) Regeneration of long spinal axons in the rat. J Neurocytol 13:165–182
Shieh JY, Leong SK, Wong WC (1983) Origin of the rubrospinal tract in neonatal, developing, and mature rats. J Comp Neurol 214:79–86
Shinoda Y, Ghez C, Arnold A (1977) Spinal branching of rubrospinal axons in the cat. Exp Brain Res 30:203–218
Tetzlaff W, Alexander SW, Miller FD, Bisby MA (1991) Responses of facial and rubrospinal neurons to axotomy: changes in mRNA expression for cytoskeletal proteins and GAP-43. J Neurosci 11:2528–2544
Tseng G-F, Prince DA (1996) Structural and functional alterations in rat corticospinal neurons after axotomy. J Neurophysiol 75:248–267
Tseng G-F, Parada I, Prince DA (1991) Doublelabelling with rhodamine beads and biocytin: a technique for studying corticospinal and other projection neurons in vitro. J Neurosci Methods 37:121–131
Tseng G-F, Shu J, Huang S-J, Wang Y-J (1995) A time-dependent loss of retrograde transport ability in distally axotomized rubrospinal neurons. Anat Embryol 191:243–249
Tseng G-F, Wong Y-J, Lai Q-C (1996) Perineuronal microglial reactivity following proximal and distal axotomy of rat rubrospinal neurons. Brain Res 715:32–43
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tseng, GF., Wang, YJ. & Hu, ME. Axotomy affects the retrograde labeling of cervical and lumbar-cord-projecting rubrospinal neurons differently. Anat Embryol 194, 457–464 (1996). https://doi.org/10.1007/BF00185993
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00185993