Abstract
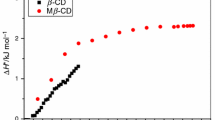
The energetics of dissociation of bovine insulin in aqueous solution have been investigated by sensitive dilution microcalorimetry. Cyclodextrins increase dissociation of insulin oligomers in a manner consistent with their interaction with protein side chains. For example, assuming monomer-dimer equilibrium, in the absence of cyclo-dextrins the calorimetric dilution data (25 °C, pH 2.5) indicate a dimer dissociation constant (Kdiss) of about 12 µM and an endothermic dissociation enthalpy (ΔHdiss) of +41 kJ mol−1. Addition of methyl-β-cyclodextrin (up to 200 mm) makes dissociation significantly more endothermic (ΔHdiss = 79 kJ mol−1) and reduces the apparent dimer dissociation constant by more than two orders of magnitude (Kdiss ≈ 1.7 mm). Qualitatively similar results are observed with α-cyclodextrin and other β-cyclodextrin derivatives. Cyclodextrin-induced insulin dissociation is also observed at pH 7.4.
Similar content being viewed by others
References
Bi RC, Dauter Z, Dodson E, Dodson G, Giordano F, Reynolds C (1984) Insulin's structure as a modified and monomeric molecule. Biopolymers 23: 391–395
Blundell TL, Dodson G, Hodgson D, Mercola D (1972) Insulin: the structure in the crystal and its reflection in chemistry and biology. Adv Prot Chem 26: 279–402
Brewster ME, Hora MS, Simpkins JW, Bodor N (1991) Use of 2-hydroxypropyl-\-cyclodextrin as a solubilizing and stabilizing excipient for protein drugs. Pharm Res 8: 792–795
Camilleri P, Haskins NJ, Howlett DR (1994) β-cyclodextrin interacts with the Alzheimer amyloid β-A4 peptide. FEBS Lett 341: 256–258
Cooper A (1992) Effect of cyclodextrins on the thermal stability of globular proteins. J Amer Chem Soc 114: 9208–9209
Cooper A, Johnson CM (1994) Isothermal titration microcalorimetry. In: Jones C, Mulloy B, Thomas AH (eds) Microscopy, optical spectroscopy, and macroscopic techniques, methods in molecular biology, Vol 22. Humana Press, Totowa, NJ, pp 137 -150
Cooper A, MacNicol DD (1978) Chiral host-guest complexes: interaction of α-cyclodextrin with optically active benzene derivatives. J Chem Soc Perkin II, 1978: 761–763
Cooper A, McAuley-Hecht KE (1993) Microcalorimetry and the molecular recognition of peptides and proteins. Phil Trans R Soc Lond A 345: 23–35
Gill SC, Hippel PH von (1989) Calculation of protein extinction coefficients from amino acid sequence data. Analyt Biochem 182: 319–326
Horsky J, Pitha J (1994) Inclusion complexes of proteins: interaction of cyclodextrins with peptides containing aromatic amino acids studied by competitive spectrophotometry. J Inclusion Phenomena and Mol Recognition in Chemistry 18: 291–300
Porter RR (1953) Partition chromatography of insulin and other proteins. Biochem J 53: 320–328
Wiseman T, Williston S, Brandts JF, Lin L-N (1989) Rapid measurement of binding constants and heats of binding using a new titration calorimeter. Analyt Biochem 179: 131–137
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lovatt, M., Cooper, A. & Camilleri, P. Energetics of cyclodextrin-induced dissociation of insulin. Eur Biophys J 24, 354–357 (1996). https://doi.org/10.1007/BF00180377
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00180377