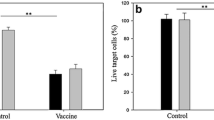
The successful application of a non-oncogenic virus, Newcastle disease virus (NDV), which can be used to modify a highly metastatic tumor to become more immunogenic is reported. Such NDV modified tumor cells were found to be effective as tumor vaccine for anti-metastatic therapy in combination with surgical removal of the primary tumor. The protection in the animals seen after this treatment is paralleled by an establishment of specific systemic anti-tumor immunity. This protective immunity depended on recognition of a distinct tumor antigen. The therapy protocol also worked in animals bearing the plastic adhesive variant ESb-MP. It did not work, however, when using an immune escape variant not expressing a specific tumor antigen]
Similar content being viewed by others
References
Altevogt, P., Leidig, S., and Heckl-Östreicher, B., 1984, Resistance of metastatic tumor variants to tumor-specific cytotoxic T lymphocytes not due to defects in expression of restricting major histocompatibility complex molecules in routine cells. Cancer Research, 44, 5305–5313.
Altevogt, P., von Hoegen, P., and Schirrmacher, V., 1986, Immunoresistant metastatic tumor variants can reexpress their tumor antigen by treatment with DNA methylation inhibiting agents. International Journal of Cancer, 38, 707–711.
Bosslet, K., and Schirrmacher, V., 1982, High-frequency generation of new immunoresistant tumor variants during metastasis of a cloned murine tumor line (ESb). International Journal of Cancer, 29, 195–202.
Bosslet, K., Schirrmacher, V., and Shantz, G., 1979, Tumor metastases and cell-mediated immunity in a model system in DBA/2 mice. VI. Similar specificity patterns of protective anti-tumor immunity in vivo and of cytolytic T cells in vitro. International Journal of Cancer, 24, 303–313.
Cassel, W. A., and Garrett, R. E., 1965, Newcastle disease virus as an antineoplastic agent. Cancer, 18, 863–868.
Cassel, W. A., Murray, D. A., Torbin, A. H., Olkowski, Z. L., and Moore, M. E., 1977, Viral oncolysate in the management of malignant melanoma. I. Preparation of the oncolysate and measurement of immunologic responses. Cancer, 40, 672–679.
Cassel, W. A., Murray, D. R., and Phillips, H. S., 1983, A phase II study on the postsurgical management of stage I I malignant melanoma with a Newcastle disease virus oncolysate. Cancer, 52, 856–860.
Fogel, M., Altevogt, P., and Schirrmacher, V., 1983, Metastatic potential severely altered by changes in tumor cell adhesiveness and cell surface sialylation. Journal of Experimental Medicine, 157, 371–376.
Heicappell, R., Schirrmacher, V., von Hoegen, P., Ahlert, T., and Appelhans, B., 1986, Prevention of metastatic spread by postoperative immunotherapy with virally modified autologous tumor cells. I. Parameters for optimal therapeutic effects. International Journal of Cancer, 37, 569–577.
Ito, Y., Nagai, Y., and Maeno, K., 1982, Interferon production in mouse spleen cells and mouse fibroblasts (L cells) stimulated by various strains of Newcastle disease virus. Journal of Genetic Virology, 62, 349–352.
Kerbel, R. S., Twiddy, R. R., and Robertson, D. M., 1978, Induction of a tumor with greatly increased metastatic growth potential by injection of cells from a low-metastatic H-2 heterozygous tumor cell line into an H-2 incompatible parental strain. International Journal of Cancer, 22, 583–594.
Kobayashi, H., 1979, Viral xenogenization of intact tumor cells. Advances in Cancer Research, 30, 279–299.
Russell, P. H., and Alexander, D. J., 1983, Antigenic variation of Newcastle disease virus strains detected by monoclonal antibodies. Archives of Virology, 75, 243–253.
Schirrmacher, V., Shantz, G., Clauer, K., Komitowski, D., Zimmermann, H.-P., and Lohmann-Matthes, M. L., 1979, Tumor metastases and cell-mediated immunity in a model system in DBA/2 mice. I. Tumor invasiveness in vitro and metastases formation in vivo. International Journal of Cancer, 23, 233–244.
Schirrmacher, V., Bosslet, K., Shantz, G., Clauer, K., and Hübsch, D., 1979, Tumor metastases and cell-mediated immunity in a model system in DBA/2 mice. IV. Antigenic differences between a metastasizing variant and the parental tumor line revealed by cytotoxic T-lymphocytes. International Journal of Cancer, 23, 245–252.
Schirrmacher, V., Ahlert, T., Heicappell, R., Appelhans, B., and von Hoegen, P., 1986, Successful application of non oncogenic viruses for antimetastatic cancer immunotherapy. Cancer Reviews, 5 (in the press).
von Hoegen, P., and Schirrmacher, V., 1986, Specific T cell responses to chemically induced tumors analysed by limiting dilution analysis. Viral xenogenization increases the frequency of cytotoxic T lymphocyte precursors specific for the tumor associated transplantation antigen. (Submitted for publication.)
Wolf, A., Barfoot, R. K., and Johnson, R. A., 1972, Xenogeneic recognition of tumor specific plasma membrane antigens derived from murine lymphoma cells. Immunology, 22, 485–491.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schirrmacher, V., Heicappell, R. Prevention of metastatic spread by postoperative immunotherapy with virally modified autologous tumor cells. II. Establishment of specific systemic anti-tumor immunity. Clin Exp Metast 5, 147–156 (1987). https://doi.org/10.1007/BF00058060
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00058060