Abstract
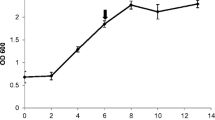
Regulation of wound-inducible 1-aminocyclopropane-1-carboxylic acid (ACC) synthase expression was studied in tomato fruit (Lycopersicon esculentum cv. Pik-Red). A 70 base oligonucleotide probe homologous to published ACC synthase cDNA sequences was successfully used to identify and analyze regulation of a wound-inducible transcript. The 1.8 kb ACC synthase transcript increased upon wounding the fruit as well as during fruit ripening. Salicylic acid, an inhibitor of wound-responsive genes in tomato, inhibited the wound-induced accumulation of the ACC synthase transcript. Further, polyamines (putrescine, spermidine and spermine) that have anti-senescence properties and have been shown to inhibit the development of ACC synthase activity, inhibited the accumulation of the wound-inducible ACC synthase transcript. The inhibition by spermine was greater than that caused by putrescine or spermidine. The transcript level of a wound-repressible glycine-rich protein gene and that of the constitutively expressed rRNA were not affected as markedly by either salicylic acid or polyamines. These data suggest that salicylic acid and polyamines may specifically regulate ethylene biosynthesis at the level of ACC synthase transcript accumulation.
Similar content being viewed by others
References
Adams DO, Yang SF: Ethylene biosynthesis: Identification of 1-aminocyclopropane-1-carboxylic acid as an intermediate in the conversion of methionine to ethylene. Proc Natl Acad Sci USA 76: 170–174 (1979).
Apelbaum A, Burg SP: Effect of ethylene on cell division and deoxyribonucleic acid synthesis in Pisum sativum. Plant Physiol 50: 117–124 (1972).
Apelbaum A, Burgoon AC, Anderson JD, Lieberman M, Ben-Arie R, Mattoo AK: Polyamines inhibit biosynthesis of ethylene in higher plant tissue and protoplasts. Plant Physiol 68: 453–456 (1981).
Apelbaum A, Goldlust A, Icekson I: Control by ethylene of arginine decarboxylase activity in pea seedlings and its implication for hormonal regulation of plant growth. Plant Physiol 79: 635–640 (1985).
Ben-Arie R, Lurie S, Mattoo AK: Temperature dependent inhibitory effects of calcium and spermine on ethylene biosynthesis in apple discs correlate with changes in microsomal membrane viscosity. Plant Sci Lett 24: 239–247 (1982).
Boller T, Herner RC, Kende H: Assay for and enzymatic formation of an ethylene precursor, 1-aminocyclopropane-1-carboxylic acid. Planta 145: 293–304 (1979).
Boller T: Ethylene in pathogenesis and disease resistance. In: Mattoo AK, Suttle JC (eds) The Plant Hormone Ethylene, pp. 293–314. CRC Press, Boca Raton, FL (1991).
Brady CJ: Fruit ripening. Annu Rev Plant Physiol 38: 155–178 (1987).
Burg SP, Burg EA: The interaction between auxin and ethylene and its role in plant growth. Proc Natl Acad Sci USA 55: 262–269 (1966).
Chirgwin JM, Przybyla AE, MacDonald RJ, Rutter WJ: Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry 18: 5294–5299 (1979).
Dibble ARG, Davies PJ, Mutschller MA: Polyamine content of long-keeping Alcobaca tomato fruit. Plant Physiol 86: 338–340 (1988).
Doherty HM, Selvendram RR, Bowles DJ: The wound response of tomato plants can be inhibited by aspirin and related hydroxy-benzoic acids. Physiol Mol Plant Pathol 33: 377–384 (1988).
Evans PT, Malmberg RL: Do polyamines have roles in plant development? Annu Rev Plant Physiol Plant Mol Biol 40: 235–269 (1989).
Even-Chen Z, Mattoo AK, Goren R: Inhibition of ethylene biosynthesis by aminoethoxyvinylglycine and by polyamines shunts label from [3,4-14C] methionine into sperimidine in aged orange peel discs. Plant Physiol 69: 385–388 (1982).
Flores HE, Arteca RN, Shannon JC: Polyamines and Ethylene: Biochemistry, Physiology, and Interactions. American Society of Plant Physiologists, Washington, DC (1990).
Fuhrer J: Ethylene biosynthesis and cadmium toxicity in leaf tissue of beans (Phaseolus vulgaris L). Plant Physiol 70: 162–167 (1982).
Grierson D: Gene expression in ripening tomato fruit. CRC Crit Rev Plant Sci 3: 113–132 (1985).
Hyodo H: Stress/wound ethylene. In: Mattoo AK, Suttle JC (eds) The Plant Hormone Ethylene, pp. 43–63. CRC Press, Boca Raton, FL (1991).
Kende H, Boller T: Wound ethylene and 1-aminocyclopropane-1-carboxylate synthase in ripening tomato fruit. Planta 151: 476–481 (1981).
Lawyer FC, Stoffel S, Saiki RK, Mayambo KB, Drummond R, Gelfand DH: Isolation, characterization, and expression in E. coli of the DNA polymerase gene from the extreme thermophile, Thermus aquaticus. J Biol Chem 264: 6427–6437 (1989).
Leslie CA, Romani RJ: Salicylic acid: A new inhibitor of ethylene biosynthesis. Plant Cell Rep 5: 144–146 (1986).
Lieberman M: Biosynthesis and action of ethylene. Annu Rev Plant Physiol 30: 533–591 (1979).
Lincoln JE, Cordes S, Read E, Fischer RL: Regulation of gene expression by ethylene during Lycopersicon esculentum (tomato) fruit development. Proc Natl Acad Sci USA 84: 2793–2797 (1987).
Malamy J, Carr JP, Klessig DF, Raskin I: Salicylic acid: a likely endogenous signal in the resistance response of tobacco to viral infection. Science 250: 1002–1004 (1990).
Maniatis T, Fritsch EF, Sambrook J: Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY (1982).
Mattoo AK, Adams DO, Patterson GW, Lieberman M: Inhibition of 1-aminocyclopropane-1-carboxylic acid synthase by phenothiazines. Plant Sci Lett 28: 173–179 (1982).
Mattoo AK, Aharoni N: Ethylene and plant senescence. In: Nooden L, Leopold AC (eds) Senescence and Aging in Plants, pp. 241–280. Academic Press, London (1988).
Mattoo AK, Anderson JD: Wound-induced increase in 1-aminocylcopropane-1-carboxylate synthase activity: Regulatory aspects and membrane association of the enzyme. In: Fuchs Y, Chalutz E (eds) Ethylene: Biochemistry, Physiological and Applied aspects, pp. 139–147. Martinus Nijhoff/Dr W. Junk Publishers, Amsterdam (1984).
Mattoo AK, Suttle JC: The Plant Hormone Ethylene. CRC Press, Boca Raton, FL (1991).
Mattoo AK, White B: Regulation of ethylene biosynthesis. In: Mattoo AK, Suttle JC (eds) The Plant Hormone Ethylene, pp. 21–42. CRC Press, Boca Raton, FL, (1991).
Mehta AM, Jordan RL, Anderson JD, Mattoo AK: Identification of a unique isoform of 1-aminocyclopropane-1-carboxylic acid synthase by monoclonal antibody. Proc Natl Acad Sci USA 85: 8810–8814 (1988).
Metraux JP, Signer H, Ryals J, Ward E, Wyss-Benz M, Gaudin J, Raschdorf K, Schmid E, Blum W, Inverardi B: Increase in salicylic acid at the onset of systemic acquired resistance in cucumber. Science 250: 1004–1006 (1990).
Minocha SC, Papa NS, Khan AJ, Samuelsen AI: Polyamines and somatic embryogenesis in carrot. III. Effects of methylglyoxal bis(guanylhydrazone). Plant Cell Physiol 32: 395–402 (1991).
Nakajima N, Mori H, Yamazaki K, Imaseki H: Molecular cloning and sequence of a complementary DNA encoding 1-aminocyclopropane-1-carboxylate synthase induced by tissue wounding. Plant Cell Physiol 31: 1021–1029 (1990).
Olson DC, White JA, Edelman L, Harkins RN, Kende H: Differential expression of two genes for 1-aminocyclopropane-1-carboxylate synthase in tomato fruits. Proc Natl Acad Sci USA 88: 5340–5344 (1991).
Parsons BL, Mattoo AK: Wound regulated accumulation of specific transcripts in tomato fruit: interactions with fruit development, ethylene and light. Plant Mol Biol 17: 453–464 (1991).
Pech JC, Latche A, Larrigaudiere C, Reid MS: Control of early ethylene synthesis in pollinated petunia flowers. Plant Physiol Biochem 25: 431–437 (1987).
Roberts DR, Walker MA, Thompson JE, Dumbroff EB: The effects of inhibitors of polyamine and ethylene biosynthesis on senescence, ethylene production and polyamine levels in cut carnation flowers. Plant Cell Physiol 25: 315–322 (1984).
Sacher JA: Senescence and postharvest physiology. Annu Rev Plant Physiol 24: 197–224 (1973).
Sato T, Oeller PW, Theologis A: The 1-aminocyclopropane-1-carboxylate synthase of Cucurbita. Purification, properties, expression in Escherichia coli, and primary structure determination by DNA sequence analysis. J Biol Chem 266: 3752–3759 (1991).
Sato T, Theologis A: Cloning the mRNA encoding 1-aminocyclopropane-1-carboxylate synthase, the key enzyme for ethylene biosynthesis in plant. Proc Natl Acad Sci USA 86: 6621–6625 (1989).
Saftner RA, Baldi BG: Polyamine levels and tomato fruit development: possible interaction with ethylene. Plant Physiol 92: 547–550 (1990).
Scalet M, Federico R, Angelini R: Time course of diamine oxidase and peroxidase activities, and polyamine changes after mechanical injury of chick-pea seedlings. J Plant Physiol 137: 571–575 (1991).
Sexton R, Roberts JA: Cell biology of abscission. Annu Rev Plant Physiol 33: 133–162 (1982).
Smith TA: Polyamines. Annu Rev Plant Physiol 36: 117–143 (1985).
Smith TA, Marshall JHA: The di- and polyamine oxidases of plants. In: Zappia V, Pegg AE (eds) Progress in Polyamine Research, pp. 573–587. Plenum Press, New York (1988).
Southern EM: Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol 98: 503–517 (1975).
Suttle JC: Effect of polyamines on ethylene production. Phytochemistry 20: 1477–1480 (1981).
Taylorson RB, Hendricks SB: Dormancy in seeds. Annu Rev Plant Physiol 28: 331–354 (1977).
Vanden Driessche TH, Kevers C, Collet M, Gaspar TH: Acetabularia mediterranea and ethylene: production in relation with development, circadian rhythms in emission, and response to external application. J Plant Physiol 133: 635–639 (1988).
Van Der Straeten D, Van Wiemeersch L, Goodman HM, Van Montagu M: Cloning and sequence of two different cDNAs encoding 1-aminocyclopropane-1-carboxylate synthase in tomato. Proc Natl Acad Sci USA 87: 4859–4863 (1990).
Watson JC, Thompson WF: Purification and restriction endonuclease analysis of plant nuclear DNA. In: Weissbach A, Weissbach H (eds) Methods for Plant Molecular Biology, pp. 57–75. Academic Press, New York (1988).
Yang SF, Hoffman NE: Ethylene biosynthesis and its regulation in higher plants. Annu Rev Plant Physiol 35: 155–189 (1984).
Yip WK, Dong JG, Kenny JW, Thompson GA, Yang SF: Characterization and sequencing of active site of 1-aminocyclopropane-1-carboxylate synthase. Proc Natl Acad Sci USA 87: 7930–7934 (1990).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Li, N., Parsons, B.L., Liu, D. et al. Accumulation of wound-inducible ACC synthase transcript in tomato fruit is inhibited by salicylic acid and polyamines. Plant Mol Biol 18, 477–487 (1992). https://doi.org/10.1007/BF00040664
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00040664