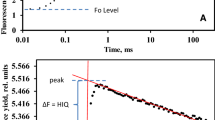
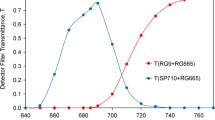
Abstract
A newly developed modulation fluorometer is described which operates with 1 μsec light pulses from a light-emitting diode (LED) at 100 KHz. Special amplification circuits assure a highly selective recording of pulse fluorescence signals against a vast background of non-modulated light. The system tolerates ratios of up to 1:107 between measuring light and actinic light. Thus it is possible to measure the “dark fluorescence yield” and record the kinetics of light-induced changes. A high time resolution allows the recording of the rapid relaxation kinetic following a saturating single turnover flash. Examples of system performance are given. It is shown that following a flash the reoxidation kinetics of photosystem II acceptors are slowed down not only by the inhibitor DCMU, but by a number of other treatments as well. From a light intensity dependency of the induction kinetics the existence of two saturated intermediate levels (I1 and I2) is apparent, which indicates the removal of three distinct types of fluorescence quenching in the overall fluorescence rise from F0 to Fmax.
Similar content being viewed by others
Abbreviations
- QA and QB :
-
consecutive electron acceptors of photosystem II
- PS II:
-
photosystem II
- P 680:
-
reaction center chlorophyll of photosystem II
- F0 :
-
minimum fluorescence yield following dark adaptation
- Fmax :
-
maximum fluorescence yield
- DCMU:
-
3-(3, 4-dichlorophenyl)-1, 1-dimethyl-urea
- DCCD:
-
N,N′-dicyclohexylcarbodiimide
- PQ:
-
plastoquinone
- DAD:
-
diaminodurene
References
Amesz J and Duysens LNM (1977) In primary processes of photosynthesis (Barber J, ed.) pp. 149–185 Amsterdam: Elsevier
Azzi A, Casey RP and Nalecz MJ (1984) Biochim Biophys Acta 768:149–185
Bouges-Bocquet B (1973) Biochim Biophys Acta 314:250–256
Bowes JM and Crofts AR (1980) Biochim Biophys Acta 590:373–384
Bradbury M and Baker NR (1981) Biochim Biophys Acta 63:542–551
Bradbury M and Baker NR (1984) Biochim Biophys Acta 765:275–281
Butler WL (1972) Proc Nat Acad Sci US 69:3420–3422
Crofts AR and Wraight CA (1983) Biochim Biophys Acta 726:149–185
Delosme R (1967) Biochim Biophys Acta 143:108–128
Den Haan GA, Gorter de Vries H and Duysens LNM (1976) Biochim Biophys Acta 430:265–281
Dietz K-J, Schreiber U and Heber U (1985) Planta, in press
Duysens LNM and Sweers HE (1963) In studies on microalgae and photosynthetic bacteria, pp. 353–372. Tokyo: University of Tokyo Press
Duysens LNM, den Haan GA and van Best JA (1975) In Proc 3rd Int Congr Photosynth (Avron M, ed.) Vol 1, pp. 1–12. Amsterdam. Elsevier
Heber U and Santarius KA (1970) Z Naturforsch 25b:718–728
Heber U (1973) Biochim Biophys Acta 305:140–152
Jensen RG and Bassham JA (1966) Proc Nat Acad Sci US 56:1095–1101
Joliot A (1974) In Proc 3rd Congr Photosynth Res, Rehovot (Avron M, ed.) Vol 1, pp. 315–322, Elsevier: Amsterdam
Kautsky H, Appel W and Amann (1960) Biochem Z 332:277–292
Klimov VV, Klevanik AV, Shuvalov VA and Krasnovskii AA (1977) FEBS Lett 82:183–186
Krause GH, Briantais JM and Vernotte C (1982) Biochim Biophys Acta 679:116–124
Krause GH and Weis E (1984) Photosynth Res 5:139–187
Kyle DJ, Ohad I and Arntzen CJ (1984) Proc Nat Acad Sci US 81:4070–4074
Laasch H, Schreiber U and Urbach W (1983) FEBS Lett 159:275–279
Lavergne J (1982) Biochim Biophys Acta 682:345–353
Lavorel J and Etienne AL (1977) In primary processes of photosynthesis (Barber J, ed.) pp. 203–268. Amsterdam: Elsevier
Mauzerall D (1972) Proc Nat Acad Sci US 69:1358–1362
Papageorgiou G (1975) In bioenergetics of photosynthesis. (ed. Govindjee) pp. 319–371. New York: Academic Press
Quick WP and Horton P (1984) Proc R Soc Lond B 220:371–382
Renger G and Schreiber U (1985) In light emissions by plants and bacteria (Govindjee, Amesz J and Fork DC, eds.) New York: Academic Press, in press
Rienits KG, Hardt H and Avron M (1974) Eur J Biochem 43:291–298
Sane PV, Johanningmeier U and Trebst A (1979) FEBS Lett 108:136–140
Schreiber U and Avron M (1979) Biochim Biophys Acta 546:436–447
Schreiber U (1983) Photosynth Res 4:361–373
Schreiber U (1984) Biochim Biophys Acta 767:70–79
Schreiber U, Bilger W and Schliwa U (1985) Photosynth Res, in press
Solioz M (1984) Trends Biochem Sci 9:309–312
Van Best (1977) Doctoral Thesis, State University of Leiden, the Netherlands
Van Best JA and Duysens LNM (1977) Biochim Biophys Acta 459:187–206
Velthuys B and Amesz J (1974) Biochim Biophys Acta 333:85–94
Velthuys BR (1980) Ann Rev Plant Physiol 31:545–567
Velthuys BR (1981) FEBS Lett 126:277–281
Vermaas WFJ and Govindjee (1981) Photochem Photobiol 34:775–793
Vernotte C, Etienne AL and Briantais J-M (1979) Biochim Biophys Acta 545: 519–527
Yamashita T and Butler WL (1968) Plant Physiol 43:2037–2040
Zankel Kl (1973) Biochim Biophys Acta 325:138–148
Author information
Authors and Affiliations
Additional information
Dedicated to Prof. L.N.M. Duysens on the occasion of his retirement.
Rights and permissions
About this article
Cite this article
Schreiber, U. Detection of rapid induction kinetics with a new type of high-frequency modulated chlorophyll fluorometer. Photosynth Res 9, 261–272 (1986). https://doi.org/10.1007/BF00029749
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00029749