Abstract
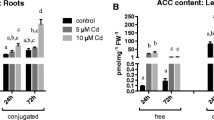
Maize plants, grown in aerated solution cultures, were exposed, at different growth stages, to ACC (1-aminocyclopropane-1-carboxylic acid) applied through the roots for up to 9 d. Total uptake of ACC increased with seedling size. During ACC treatment, ethylene evolution, by the shoots, proceeded at an almost constant rate per unit fresh weight that was up to 40-fold faster than that of untreated plants. This stimulation extended several days beyond the period of ACC uptake. The effects on growth and development were assessed when plants were 50–52-d old. ACC application shortened certain stem internodes, leaf-sheaths and laminae. The location of these effects depended on the time of application. The greatest shortening was induced by application, at the 4-leaf stage (10 d-old), prior to elongation of the cone of the shoot apex. This is ascribed to effects on meristematic tissue, in addition to those on elongating cells. An unexpected response to ACC treatment, at the 4-leaf stage, was an increase of up to four leaf-bearing stem nodes compared to untreated plants. This resulted in a parallel elevation of the uppermost ear-bearing axillary shoot to higher nodal positions. The length of leaves high in the canopy (nodes 11–16) was promoted by treating seedlings with ACC. The only clear effect of the ACC treatments on emergent axillary shoots per se was a retardation of silk elongation.
Similar content being viewed by others
References
Allan Jones C (1985) C4 Grasses and Cereals. Growth, Development and Stress Response. New York: John Wiley and Sons
Amrhein N, Forreiter C, Kionka C, Skorupka H and Tophof S (1987) Metabolism and compartmentation of 1-aminocyclopropane-1-carboxylic acid in plant cells. In: K Schreiber, HR Schütte and G Sembdner eds. Conjugated Plant Hormones, Structure, Metabolism and Function, 102–108. VEB Deutscher Verlag der Wissenschaften, Berlin
Barber DA and Lee RB (1974) The effect of micro-organisms on the absorption of manganese by plants. New Phytol 73: 97–102
Bergner Ch, Stock M, Giese M, Schütte HR and Sembdner G (1987) Long term fate of 1-(malonylamino)[2,3-14C]-cyclopropane-1-carboxylic acid (14C-MACC) in pea and tomato plants. In: K Schreiber, HR Schütte and G Sembdner eds. Conjugated Plant Hormones, Structure, Metabolism and Function, 118–122. VEB Deutscher Verlag der Wissenschaften, Berlin
Bonhomme R, Derieux M, Kiniry JR, Edmeades GO and Ozier-Lafontaine H (1991) Maize leaf number sensitivity in relation to photoperiod in multi-location field trials. Agron J 83: 153–157
Bonnett OT (1940) Development of the staminate and pistillate inflorescences of sweet corn. J Agric Res 60: 25–37
Bouzayen M, Latché A, Alibert G and Pech J-C (1988) Intracellular sites of synthesis and storage of 1-(malonylamino)cyclopropane-1-carboxylic acid and Acer pseodoplatanus cells. Plant Physiol 88: 613–617
Bradford KJ and Yang SF (1980) Xylem transport of 1-aminocyclopropabe-1-carboxylic acid, an ethylene precursor, in waterlogged tomato plants. Plant Physiol 65: 322–326
Cheng PC, Greyson RI and Walden DB (1983) Organ initiation and the development of unisexual flowers in the tassel and ear of Zea mays. Am J Bot 70: 450–462
Coupland D and Jackson MB (1991) Effects of mecoprop (an auxin analogue) on ethylene evolution and epinasty in two biotypes of Stellaria media. Ann Bot 68: 167–172
Cockshull KE and Horridge JS (1978) 2-Chloroethylphosphonic acid and flower initiation by Chrysanthemum morifolium Ramat. in short days and in long days. J Hort Sci 53: 85–90
Cox WJ and Andrade HF (1988) Growth, yield and yield components of maize as influenced by ethephon. Crop Sci 28: 536–542
Damptey HB and Aspinal D (1976) Water deficit and inflorescence development in Zea mays L Ann Bot 40: 23–35
Dunlap JR (1988) Regulation of ACC-dependent ethylene production by excised leaves from normal and albino Zea mays L. seedlings. J Exp Bot 39: 1079–1089
Earley EB and Slife FW (1969) Effect of ethrel on growth and yield of corn. Agron J 61: 821–823
Finlayson SA, Foster KR and Reid DM (1991) Transport and metabolism of 1-aminocyclopropane-1-carboxylic acid in sun-flower (Helianthus annuus L.) seedlings. Plant Physiol 96: 1360–1367
Fritz VA, Hebel JB and Borowski AM (1991) Sweetcorn genotypes versus ethephon in relation to yield components. Agron J 83: 991–995
Irish EE and Nelson TM (1991) Identification of multiple stages in the conversion of maize meristems from vegetative to floral development. Development 112: 891–898
Jackson MB (1990) Hormonal and developmental changes in plants subjected to submergence or waterlogging. Aquatic Bot 38: 49–72
Jackson MB (1991) Ethylene in root growth and development. In: AK Mattoo and JC Suttle eds. The Plant Hormone Ethylene, 159–181. CRC Press, Boca Raton
Jackson MB, Drew MC and Giffard SC (1981) Effects of applying ethylene to the root system of Zea mays on growth and nutrient concentration in relation to flooding tolerance. Physiol Plant 52: 23–28
Kiesselbach TA (1949) The Structure and Reproduction of Corn. University of Nebraska College of Agriculture, Agricultural Experiment Station Research Bulletin 161
Konsler JV and Grabau LJ (1989) Ethephon as a morphological regulator for corn. Agron J 81: 849–852
Lürssen K and Konze J (1984) Relationship between ethylene production and plant growth after application of ethylene releasing plant growth regulators. In: JA Roberts and GA Tucker eds. Ethylene and Plant Development, 363–372. Butterworths, London
Norberg OS, Mason SC and Lowry SR (1988) Ethephon influence on harvestable yield, grain quality and lodging of corn. Agron J 80: 768–772
Sass JE and Loeffel FA (1959) Development of axillary buds in maize in relation to barrenness. Agron J 51: 484–486
Van Andel OM and Verkerke DR (1978) Stimulation and inhibition of ethephon of stem and leaf growth of some Gramineae at different stages of development. J Exp Bot 29: 639–651
Ververidis P and John P (1991) Complete recovery in vitro of ethylene-forming enzyme activity. Phytochem 30: 725–727
Whalen MC (1988) The effect of mechanical impedance on ethylene production by maize roots. Can J Bot 66: 2139–2142
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pinthus, M.J., Jackson, M.B. Effects of ACC (1-aminocyclopropane-1-carboxylic acid) applied through the roots of maize seedlings on vegetative and early reproductive development of the shoots. Plant Growth Regul 14, 193–202 (1994). https://doi.org/10.1007/BF00024793
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00024793