Abstract
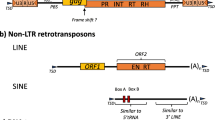
An analysis of Arabidopsis thaliana heterochromatic regions allowed the identification of a new family of retroelements called Athila. These 10.5 kb elements, representing ca. 0.3% of the genome, present several features of retrotransposons and retroviruses. Athila elements are flanked by 1.5 kb long terminal repeats (LTR) that are themselves bounded by 5 bp perfect inverted repeats. These LTRs start and end with the retroviral consensus 5′TG...CA3′ nucleotides. A putative tRNA-binding site and a polypurine tract are found adjacent to the 5′ and 3′ LTR respectively. The central domain is composed of two long open reading frames (ORFs) of 935 and 694 amino acids. Despite several indications of recent transposition activity, the translation of these ORFs failed to reveal significant homology with proteins associated to retrotransposition. We suggest that the Athila family could result from the transduction and dispersion of a cellular gene by a retrotransposon.
Similar content being viewed by others
References
Bell CJ, Ecker JR: Assignment of 30 microsatellite loci to the linkage map of Arabidopsis. Genomics 19: 137–144 (1994).
Bingham PM, Zachar Z: Retrotransposons and the FB transposon from Drosophila melanogaster. In: Berg DE, Howe MM (eds) Mobile DNA, pp. 485–502. American Society for Microbiology, Washington DC (1989).
Bishop JM, Varmus HE: Functions and origins of retroviral transforming genes. In: Weiss R, Teich N, Varmus H, Coffin J (eds) RNA Tumor Viruses, pp. 246–356. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY (1985).
Bucheton A, Paro R, Sang HM, Pelisson A, Finnegan DJ: The molecular basis of I-R dysgenesis in Drosophila melanogaster: identification, cloning and properties of the I factor. Cell 38: 153–163 (1984).
Bureau TE, White E, Wessler SR: Transduction of a cellular gene by a plant retroelement. Cell 77: 479–480 (1994).
Camirand A, St-Pierre B, Marineau C, Brisson N: Occurence of a copia-like transposable element in one of the introns of the potato starch phosphorylase gene. Mol Gen Genet 224: 33–39 (1990).
Clare J, Farabaugh P: Nucleotide sequence of yeast Ty element: evidence for an unusual mechanism of gene expression. Proc Natl Acad Sci USA 82: 2829–2833 (1985).
Dessen P, Fondrat C, Valencien C, Mugnier C: BISANCE: a French service for access to biomolecular sequence databases. Cabios 6: 355–356 (1990).
Doolittle RF, Feng DF, Johnson MS, Mc Clure MA: Origins and evolutionary relationships of retroviruses. Q Rev Biol 64: 1–30 (1989).
Doyle JJ, Doyle JL: A rapid DNA isolation procedure for small quantities of fresh tissue. Phytochem Bull 19: 11–15 (1987).
Evgen'ev MB, Corces VG, Lankenau DH: Ulysses transposable element of Drosophila shows high structural similarities to functional domains of retroviruses. J Mol Biol 225: 917–924 (1992).
Grandbastien MA: Retroelements in higher plants. Trends Genet 8: 103–108 (1992).
Grandbastien MA, Spielmann A, Caboche M: Tnt1, a mobile retroviral-like transposable element of tobacco isolated by plant cell genetics. Nature 337: 376–380 (1989).
Jin YK, Bennetzen JL: Structure and coding properties of Bs1, a maize retrovirus-like transposon. Proc Natl Acad Sci USA 86: 6235–6239 (1989).
Jin YK, Bennetzen JL: Integration and nonrandom mutation of a plasma membrane proton ATPase gene fragment within the Bs1 retroelement of maize. Plant Cell 6: 1177–1186 (1994).
Johns MA, Babcock MS, Fuerstenberg SM, Fuerstenberg SI, Freeling M, Simpson RB: An unusually compact retrotransposon in maize. Plant Mol Biol 12: 633–642 (1989).
Konieczny A, Voytas DF, Cummings MP, Ausubel FM: A superfamily of Arabidopsis thaliana retrotransposons. Genetics 127: 801–809 (1991).
Koornneef M, van Eden J, Hanhart CJ, Stam P, Braaksma FJ, Feenstra WJ: Linkage map of Arabidopsis thaliana. J Hered 74: 265–272 (1983).
Loeb DD, Padgett RW, Hardies SC, Shehee WR, Comer MB, Edgell MH, Hutchison CA: The sequence of a large L1 Md element reveals a tandemly repeated 5′ end and several features found in retrotransposons. Mol Cell Biol 6: 168–182 (1986).
Lucas H, Moore G, Flavell RB: Characterization of retrotransposon-like element in wheat. Mol Cell Biol 18: 282 (1989).
Manninen I, Schulman AH: BARE-1, a copia-like retroelement in barley (Hordeum vulgare L.). Plant Mol Biol 22: 829–846 (1993).
Martinez-Zapater JM, Estelle MA, Somerville CR: A highly repeated DNA sequence in Arabidopsis thaliana. Mol Gen Genet 204: 417–423 (1986).
Miklos GLG, Yamamoto MT, Davies J, Pirotta V: Microcloning reveals a high frequency of repetitive sequences characteristic of chromosome 4 and the β-heterochromatin of Drosophila melanogaster. Proc Natl Acad Sci USA 85: 2051–2055 (1988).
Mount SM, Rubin GM: Complete nucleotide sequence of the Drosophila transposable element copia: homology between copia and retroviral proteins. Mol Cell Biol 5: 1630–1638 (1985).
Palmgren MG: Capturing of host DNA by a plant retroelement: Bs1 encodes plasma membrane H+-ATPase domains. Plant Mol Biol 25: 137–140 (1994).
Purugganan MD, Wessler SR: Molecular evolution of magellan, a maize Ty3/gypsy-like retrotransposon. Proc Natl Acad Sci USA 91: 11674–11678 (1994).
Rackwitz HR, Zehetner G, Frischauf AM, Lehrach H: Rapid restriction mapping of DNA cloned in lambda phage vector. Gene 30: 195–200 (1984).
Rogers JH: The origin and evolution of retroposons. Int Rev Cytol 93: 187–279 (1985).
Sanger F, Nicklen S, Coulson AR: DNA sequencing with chain terminating inhibitors. Proc Natl Acad Sci USA 77: 5463–5467 (1977).
Scheinker VSH, Lozovskaya ER, Bishop JG, Corces VG, Evgen'ev MB: A long terminal repeat-containing retrotransposon is mobilized during dysgenesis in Drosophila virilis. Proc Natl Acad Sci USA 87: 9615–9619 (1990).
Schwarz-Sommer Z, Leclercq L, Gobel E, Saedler H: Cin 4, an insert altering the structure of the A1 gene in Zea mays, exhibits properties of non viral retrotransposons. EMBO J 6: 3873–3880 (1987).
Shell BE, Collins JT, Elenich LA, Szurek PF, Dunnic WA: Two subfamilies of murine retrotransposon ETn sequences. Gene 86: 269–274 (1990).
Simoens CR, Gielen J, Van Montagu M, Inze D: Characterization of highly repetitive sequences of Arabidopsis thaliana. Nucl Acids Res 16: 6753–6766 (1988).
Smyth DR, Kalitsis P, Joseph JL, Sentry JW: Plant retrotransposon from Lilium henryi is related to Ty3 of yeast and the gypsy group of Drosophila. Proc Natl Acad Sci USA 86: 5015–5019 (1989).
Temin HM: Reverse transcription in the eukaryotic genome: retroviruses, pararetroviruses, retrotransposons and retrotranscripts. Mol Biol Evol 2: 455–468 (1985).
Varmus H, Brown P: Retroviruses. In: Berg DE, Howe MM (eds) Mobile DNA, pp. 53–108. American Society for Microbiology, Washington DC (1989).
Vaury C, Bucheton A, Pelisson A: The β heterochromatic sequences flanking the I elements are themselves defective transposable elements. Chromosoma 98: 215–224 (1989).
Voytas DF, Ausubel FM: A copia-like transposable element family in Arabidopsis thaliana. Nature 336: 242–244 (1988).
White SE, Habera LF, Wessler SR: Retrotransposons in the flanking regions of normal plant genes: a role for copia-like elements in the evolution of gene structure and expression. Proc Natl Acad Sci USA 91: 11792–11796 (1994).
Xiong Y, Eickbush TH: Similarity of reverse transcriptase-like sequences of viruses, transposable elements, and mitochondrial introns. Mol Biol Evol 5: 675–690 (1988).
Xiong Y, Eickbush TH: Origin and evolution of retroelements based upon their reverse transcriptase sequences. EMBO J 9: 3353–3362 (1990).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pélissier, T., Tutois, S., Deragon, J.M. et al. Athila, a new retroelement from Arabidopsis thaliana . Plant Mol Biol 29, 441–452 (1995). https://doi.org/10.1007/BF00020976
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00020976