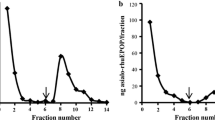
Abstract
Erythropoietin (Epo), a glycoprotein that regulates the formation of erythrocytes in mammals, was produced in cultured tobacco BY2 cells (Nicotiana tabacum L. cv. Bright Yellow 2) by introducing human Epo cDNA via Agrobacterium tumefaciens-mediated gene transfer. Epo was correctly processed and subsequently penetrated the plasma membrane of tobacco cells. However, it remained attached to the cell wall and was not released into the culture medium. Although Epo produced by tobacco cells was glycosylated with N-linked oligosaccharides, these carbohydrates were smaller than those of the recombinant Epo produced in mammalian cells. Epo produced in tobacco exhibited in vitro biological activities by inducing the differentiation and proliferation of erythroid cells. However, it had no in vivo biological activities. A lectin-binding assay indicated the lack of sialic acid residues in the N-linked oligosaccharides of Epo, suggesting that Epo was removed from the circulation before it reached erythropoietic tissues.
Similar content being viewed by others
References
An G: High efficiency transformation of cultured tobacco cells. Plant Physiol 79: 568–570 (1985).
Benvenuto E, Ordas RJ, Tavazza R, Ancora G, Biocca S: ‘Phytoantibodies’: a general vector for the expression of immunoglobulin domains in transgenic plants. Plant Mol Biol 17: 865–874 (1991).
Bevan M: Binary Agrobacterium vectors for plant transformation. Nucl Acids Res 12: 8712–8721 (1984).
Bhavanadan VP, Katlic AW: The interaction of wheat germ agglutinin with sialoglycoproteins. J Biol Chem 254: 4000–4008 (1979).
Carpita N, Sabularse D, Montezinos D, Delmer DP: Determination of the pore size of cell walls of living plant cells. Science 205: 1144–1147 (1979).
Delorme E, Lorenzini T, Griffin J, Martin F, Jacogsen F, Boone T, Elliott S: Role of glycosylation on the secretion and biological activity of erythropoietin. Biochemistry 31: 9871–9876 (1992).
Dordal MS, Wang FF, Goldwasser: The role of carbohydrate in erythropoietin action. Endocrinology 116: 2293–2299 (1985).
Drickamer K: Clearing up glycoprotein hormones. Cell 67: 1029–1032 (1991).
Driouich A, Faye L, Staehelin LA: The plant Golgi apparatus; a factory for complex polysaccharides and glycoproteins. Trends Biotechnol 18: 210–214 (1993).
Düring K, Hippe S, Kreuzaler F, Schell J: Synthesis and self-assembly of a functional monoclonal antibody in transgenic Nicotiana tabacum. Plant Mol Biol 15: 281–293 (1990).
Fukuda MN, Sasaki H, Lopez L, Fukuda M: Survival of recombinant erythropoietin in the circulation: the role of carbohydrates. Blood 73: 84–89 (1989).
Gasser CG, Fraley RT: Genetically engineering plants for crop improvements. Science 244: 1293–1299 (1989).
Goldwasser E, Gross M: Erythropoietin: assay and study of its mode of action. Meth Enzymol 37: 109–121 (1975).
Goto M, Akai K, Murakami A, Hashimoto C, Tsuda E, Ueda M, Kawanishi G, Takahashi N, Ishimoto A, Chiba H, Sasaki R: Production of recombinant human erythropoietin in mammalian cells: host-cell dependency of the biological activity of the cloned glycoprotein. Bio/Technology 6: 67–71 (1988).
Goto M, Murakami A, Akai K, Kawanishi G, Ueda M, Chiba H, Sasaki R: Characterization and use of monoclonal antibodies directed against human erythropoietin that recognize different antigenic determinants. Blood 74: 1415–1423 (1989).
Hein MB, Tang Y, McLeod DA, Janda KD, Hiatt A: Evaluation of immunoglobulins from plant cells. Biotechnol Prog 7: 455–461 (1991).
Hiatt A, Cafferkey R, Bowdish K: Production of antibodies in transgenic plants. Nature 342: 76–78 (1989).
Hiatt A, Ma JKC: Monoclonal antibody engineering in plants. FEBS Lett 307: 71–75 (1992).
Higuchi M, Oh-eda M, Kuboniwa H, Tomonoh K, Shimonaka Y, Ochi N: Role of sugar chains in the expression of the biological activity of human erythropoietin. J Biol Chem 267: 7703–7709 (1992).
Iscove NN, Sieber F, Winterhalter KH: Erythroid colony formation in cultures of mouse and human bone marrow: analysis of the requirement for erythropoietin by gel filtration and affinity chromatography on agarose-concanavalin A. J Cell Physiol 83: 309–320 (1974).
Jacobs K, Shoemaker C, Ruderdorf R, Neill SD, Kaufman RJ, Mufson A, Seehra J, Jones SS, Hewick R, Fritsch EF, Kawakita M, Shimizu T, Miyake T: Isolation and characterization of genomic and cDNA clones of human erythropoietin. Nature 313: 806–810 (1985).
Krantz SB: Erythropoietin. Blood 77: 419–434 (1991).
Lin FK, Suggs S, Lin CH, Browne JK, Smalling R, Egrie JC, Chen KK, Fox GM, Martin F, Stabinsky Z, Badrawi SM, Lai PH, Goldwasser E: Cloning and expression of the human erythropoietin gene. Proc Natl Acad Sci USA 82: 7580–7584 (1985).
Linsmaier EM, Skoog F: Organic growth factor requirements of tobacco tissue cultures. Physiol Plant 18: 100–127 (1965).
Mason HS, Kam DMK, Arntzen CJ: Expression of hepatitis B surface antigen in transgenic plants. Proc Natl Acad Sci USA 89: 11745–11749 (1992).
Matsumoto S, Ishii A, Ikura K, Ueda M, Sasaki R: Expression of human erythropoietin in cultured tobacco cells. Biosci Biotech Biochem 57: 1249–1252 (1993).
Mary F, Trimble RB, Tarentino AL, Plummer THJ: Characterization of glycoproteins and their associated oligosaccharides through the use of endoglycanases. Anal Biochem 180: 195–204 (1989).
Miyake T, Kung CKH, Goldwasser E: Purification of human erythropoietin. J Biol Chem 252: 5558–5564 (1977).
Morikawa H, Iida A, Yamada Y: Electric gene transfer into plant protoplasts and cells. In: Mizrahi A (eds) Biotechnology in Agriculture, pp. 175–202. Alan R. Liss, New York (1988).
Mosmann T: Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Meth 65: 55–63 (1983).
Nagata T, Okada K, Takebe I, Matsui C: Delivery of tobacco mosaic virus RNA into plant protoplasts mediated by reverse-phase evaporation vesicles (liposomes). Mol Gen Genet 184: 161–165 (1981).
Poretz RD, Morell AG: An expression of the topography of the saccharide binding sites of Concanavalin A and of the forces involved in complexation. Biochemistry 9: 2890–2896 (1970).
Sasaki H, Bothner B, Dell A, Fukuda M: Carbohydrate structure of erythropoietin in chinese hamster ovary cells by a human erythropoietin cDNA. J Biol Chem 262: 12059–12076 (1987).
Sasaki R, Yanagawa S, Chiba H: Isolation of human erythropoietin with monoclonal antibodies. Meth Enzymol 147: 328–340 (1987).
Schreier PH, Seftor EA, Schell J, Bohnert HJ: The use of nuclear-encoded sequences to derect the light-regulated synthesis and transport of the foreign protein into plant chloroplasts. EMBO J 4: 25–32 (1985).
Sijmons PC, Dekker BMM, Schrammeijer B, Verwoerd TC, van denElzen PJM, Hoekema A: Production of correctly processed human serum albumin in transgenic plants. Bio/Technology 8: 217–221 (1990).
Spivak JL, Hogans BB: The in vitro metabolism of recombinant erythropoietin in the rat. Blood 73: 90–99 (1989).
Toriyama K, Arimoto Y, Uchimiya H, Hinata K: Transgenic rice plants after direct gene transfer into protoplasts. Bio/Technology 6: 1072–1074 (1988).
Tsuda E, Goto M, Murakami A, Akai K, Ueda M, Kawanishi G, Takahashi N, Sasaki R, Ishihara H, Mori M, Tejima S, Endo S, Arata Y: Comparative structural study of N-linked oligosaccharides of urinary and recombinant erythropoietins. Biochemistry 27: 5646–5654 (1988).
Tsuda E, Kawanishi G, Ueda M, Masuda S, Sasaki R: The role of carbohydrate in recombinant human erythropoietin. Eur J Biochem 188: 405–411 (1990).
Vandekerckhove J, VanDamme J, VanLijsebettens M, Botterman J, DeBlock M, Vandewiele M, DeClercq A, Leemans J, VanMontagu M, Krebbers E: Enkephalins produced in transgenic plants using modified 2S seed storage proteins. Bio/Technology 7: 929–932 (1989).
Weising K, Schell J, Kahl G: Foreign genes in plants: transfer, structure, expression, and applications. Annu Rev Genet 22: 421–477 (1988).
Yamaguchi K, Akai K, Kawanishi G, Ueda M, Masuda S, Sasaki R: Effect of site-directed removal of N-glycosylation site in human erythropoietin on its production and biological properties. J Biol Chem 266: 20434–20439 (1991).
Yamamoto K, Tsuji T, Matsumoto I, Osawa T: Structural requirements for the binding of oligosaccharides and glycopeptides to immobilized wheat germ agglutinin. Biochemistry 20: 5894–5899 (1981).
Zambryski P, Tempe J, Schell J: Transfer and function of T-DNA genes from Agrobacterium Ti and Ri plasmids in plants. Cell 56: 193–201 (1989).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Matsumoto, S., Ikura, K., Ueda, M. et al. Characterization of a human glycoprotein (erythropoietin) produced in cultured tobacco cells. Plant Mol Biol 27, 1163–1172 (1995). https://doi.org/10.1007/BF00020889
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00020889