Abstract
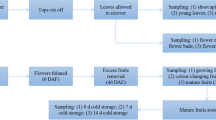
We have isolated and sequenced two cDNA clones (PM 18.2A;PM 18.2B) from Douglas fir (Pseudotsuga menziesii (Mirb.) Franco) which encode for the low-molecular-weight heat shock proteins (LMW HSPs) of 18.2 kDa. The predicted amino acid sequences of the two Douglas fir proteins are 97.5% identical. A phylogenetic tree of class I LMW HSPs showed that the PM LMW HSPs are found within a subgroup consisting exclusively of dicot species indicating that class I LMW HSPs evolved from a common ancestor predating the divergence of gymnosperms and angiosperms. Northern blots of RNA from dry, imbibed, stratified and germinated seeds revealed a notable induction of LMW HSP transcripts during post-germination and early seedling growth. Unlike previous reports, the expression of these HSPs appears to be primarily restricted to seedlings as mRNA transcripts were detected at very low levels during seed development and desiccation. Maximum induction of LMW HSPs in seedlings occurred during heat shock treatment at 38–40°C, whereas cold shock or wounding failed to induce HSP transcripts. The transcription of HSP genes is up regulated by GA, MeJA and auxin and is down regulated by ABA. Methyl jasmonate treatment induced expression of these genes in dormant seeds of Douglas fir. The expression of class I cytoplasmic LMW HSPs in seedlings and their regulation by plant growth regulators suggests specific roles in plant development other than desiccation tolerance.
Similar content being viewed by others
References
Almoguera C, Jordano J: Developmental and environmental concurrent expression of sunflower dry-seed-stored low molecular weight heat shock proteins and LEA mRNAs. Plant Mol Biol 19: 781–792 (1992).
Almoguera C, Jordano J: Differential accumulation of sunflower tetraubiquitin mRNAs during zygotic embryogenesis and developmental regulation of their heat-shock response. Plant Physiol 107: 765–773 (1995).
Altschul SF, Gish W, Miller W, Myers EW, Lipman DI: Basic local alignment search tools. J Mol Biol 215: 403–410 (1990).
Beckmann RP, Mizzen LA, Welch WJ: Interaction of Hsp 70 with newly synthesized proteins: implications for protein folding and assembly. Science 248: 850–853 (1990).
Benndorf R, Haye K, Ryazantsev S, Wieske M, Behike J, Lutsch G: Phoshorylation and supramolecular organization of murine small heat shock protein HSP25 abolish its actin polymerization-inhibiting activity. J Biol Chem 269: 20780–20784 (1994).
Berestezky V, Dathe W, Daletskaya T, Musatenko L, Sembdner G: Jasmonic acid in seed dormancy of Acer tataricum. Biochem Physiol Pflanzen 187: 13–19 (1991).
Cavener DR, Ray SC: Eukaryotic start and stop translation sites. Nucl Acids Res 19: 3185–3192 (1991).
Coca MA, Almoguera C, Jordano J: Expression obsun-flower low molecular weight heat shock proteins during embryogenesis and persistence after germination: localization and possible functional implications. Plant Mol Biol 25: 479–492 (1994).
Craig EA, Gambill BD, Nelson RJ: Heat shock proteins: molecular chaperones of protein biogenesis. Microbiol Rev 57: 402–414 (1993).
Darwish K, Wang L, Hwang CH, Apuya N, Zimmerman JL: Cloning and characterization of genes encoding low molecular weight heat shock proteins from carrot. Plant Mol Biol 16: 729–731 (1991).
DeRocher A, Helm KW, Lauzon LM, Vierling E: Expression of a conserved family of cytoplasmic low molecular weight heat shock proteins during heat stress and recovery. Plant Physiol 96: 1038–1407 (1991).
DeRocher AE, Vierling E: Developmental control of small heat shock protein expression during pea seed maturation. Plant J 5: 93–102 (1994).
Ellis RJ: Molecular chaperones: the plant connection. Science 250: 954–958 (1990).
Felsenstein J: PHYLIP-Phylogeny Interference Package (Version 3.2). Cladistics 5: 64–166 (1989).
Gaestel M, Gross B, Benndorf E, Strauss M, Schunk W-H, Draft R, Otto A, Bohm H, Stahl J, Drabsch H, Bielka H: Molecular cloning, sequencing and expression in Escherichia coli of the 25-kDa growth-related protein of Ehrlich ascites tumor and its homology to mammalian stress proteins. Eur J Biochem 179: 209–213 (1989).
Gish W, States DJ: Identification of protein coding regions by database similarity search. Nature Genet 3: 266–272 (1993).
Gurley WB, Key JL: Transcriptional regulation of the heat shock response: a plant perspective. Biochemistry 30: 1–12 (1991).
Gyorgyey J, Gartner A, Nemeth K, Magyar Z, Hirt H, Heberle-Bors E, Dudits D: Alfalfa heat shock genes are differentially expressed during somatic embryogenesis. Plant Mol Biol 16: 999–1007 (1991).
Helm KW, Abernethy RH: Heat shock proteins and their mRNAs in dry and early imbibing emryos of wheat. Plant Physiol 93: 1626–1633 (1990).
Helm KW, LaFayette PR, Nagao RT, Key JL, Vierling E: Localization of small heat shock proteins to the higher plant endomembrane system. Mol Cell Biol 13: 238–247 (1993).
Hendrick JP, Hartl F-U: Molecular chaperone functions of heat shock proteins. Annu Rev Biochem 62: 349–384 (1993).
Higgins DG, Sharp PM: Fast and sensitive multiple sequence alignments on a microcomputer. CABIOS 5: 151–153 (1989).
Howarth C: Heat shock proteins in Sorghum bicolor and Pennistetum americanum II. Stored RNA in sorghum seed and its relationship to heat shock protein synthesis during germination. Plant Cell Environ 13: 57–64 (1990).
Howarth CJ, Ougham HJ: Genes expression under temperature stress. New Phytol 125: 1–26 (1993).
Jakob U, Buchner J: Assisting spontaneity: the role of Hsp 90 and small Hsps as molecular chaperones. Trends Biochem Sci 19: 205–211 (1994).
Jakob U, Gaestel M, Engel K, Buchner J: Small heat shock proteins are molecular chaperones. J Biol Chem 268: 1517–1520 (1993).
Jorgensen JA, Nguyen H: Isolation, sequence and expression of cDNA encoding a class I heat shock protein (HSP 17.2) in maize. Plant Sci 97: 169–175 (1994).
Kang KI, Devin J, Cadepond F, Jibard N, Guiochon-Mantel A, Baulieu E-E, Catelli M-G: In vivo functional protein-protein interaction: Nuclear targeted hsp90 shifts cytoplasmic steroid receptor mutants into the nucleus. Proc Natl Acad Sci USA 91: 340–344 (1994).
Knauf U, Jakob U, Engel K, Buchner J, Gaestel M: Stress and mitogen-induced phosphorylation of the small heat shock protein Hsp25 by MAPKAP kinase 2 is not essential for chaperone properties and cellular thermoresistance. EMBO J 13: 54–60 (1994).
Krishnan HB, Pueppke SG: Heat shock triggers rapid protein phosphorylation in soybean seedlings. Biochem Biophys Res Commun 148: 762–767 (1987).
Kuznetsov VIV, Roshchupkin BV: Stress responses in Nicotiana sylvestris cells to salinity and high temperature. 2. synthesis of heat shock proteins and polypeptide phosphorylation. Russ J Plant Physiol 41: 566–572 (1994).
Lavoie JN, Hickey E, Weber LA, Landry J: Modulation of actin microfilament dynamics and fluid phase ninocytosis by phosphorylation of heat shock protein 27. J Biol Chem 268: 24210–24214 (1993).
Leadem CL: The role of plant growth regulators in the germination of forest tree seeds. In: Kossult SV, Ross SD (eds) Hormonal Control of Tree Growth, pp. 61–93. Martinus Nijhoff, Dordrecht (1987).
Leal I, Misra S, Attree SM, Fowke LC: Effect of abscisic acid, osmoticum and desiccation on 11S storage protein gene expression in somatic embryos of white spruce. Plant Sci 106: 121–128 (1995).
Leal I, Misra S: Developmental gene expression in conifer embryogenesis and germination. III. Analysis of crystalloid protein mRNAs and desiccation protein mRNAs in the developing embryo and megagametophyte of white spruce (Picea glauca (Moench) Voss). Plant Sci 88: 25–37 (1993).
Leal I, Misra S: Molecular cloning and characterization of legumin-like storage protein cDNA of Douglas fir seeds. Plant Mol Biol 21: 709–715 (1993).
Lee GJ, Pokala N, Vierling E: Structure and in vitro molecular chaperone activity of cytosolic small heat shock proteins from pea. J Biol Chem 270: 10432–10438 (1995).
Lenne C, Douce R: A low molecular mass heat shock protein is localized to higher plant mitochondria. Plant Physiol 105: 1255–1261 (1994).
Lindquist S, Craig EA: The heat shock proteins. Annu Rev Genet 22: 631–677 (1988).
McElwain EF, Spiker S: A wheat cDNA clone which is homologous to the 17 kDa heat-shock protein gene family of soybean. Nucl Acids Res 17: 1764 (1989).
Minowada G, Welch W: Variation in the expression and/or phosphorylation of the human low molecular weight stress protein during in vitro cell differentiation. J Biol Chem 270: 7047–7054 (1995).
Misra S: Conifer zygotic embryogenesis, somatic embryogenesis and seed germination: biochemical and molecular advances. Seed Sci Res 4: 357–384 (1994).
Muller-Uri F, Parthier B, Nover L: Jasmonate-induced alteration of gene expression in barley leaf segements analyzed by in vivo and in vitro protein synthesis. Planta 176: 241–247 (1988).
Nover L, Scharf K-D: Synthesis, modification and structural binding of heat-shock proteins in tomato cell cultures. Eur J Biochem 139: 303–313 (1984).
O'Connell MA: Heat shock proteins and thermotolerance. In: Basra AS (ed) Stress-Induced Gene Expression in Plants, pp. 163–183. Harwood Academic Publishers, Switzerland (1994).
Osteryoung KW, Vierling E: Dynamics of small heat shock protein distribution within the chloroplasts of higher plants. J Biol Chem 269: 28676–28682 (1994).
Parsell DA, Lindquist S: The function of heat shock proteins in stress tolerance: degradation and reactivation of damaged proteins. Annu Rev Genet 27: 437–496 (1993).
Pratt WB: The role of heat shock proteins in regulating the function, folding and trafficking of the glucocorticoid receptor. J Biol Chem 268: 21455–21458 (1993).
Reinbothe S, Mollenhauer B, Reinbothe C: JIPs and RIPs: The regulation of plant gene expression by jasmonates in response to environmental cues and pathogens. Plant Cell 6: 1197–1209 (1994).
Reinbothe S, Reinbothe C, Lehmann J, Parthier B: Differential accumulation of methyl jasmonate-induced mRNAs in response to abscisic acid and desiccation in barley (Hordeum vulgare). Physiol Plant 86: 49–56 (1992).
Reinbothe S, Reinbothe C, Parthier B: Methyl jasmonate represses translation initiation of a specific set of mRNAs in barley. Plant J 4: 459–467 (1993).
Reinbothe S, Reinbothe C, Parthier B: Methyl jasmonateregulated translation of nuclear-encoded chloroplast proteins in barley. J Biol Chem 268: 10606–10611 (1993).
Sambrook J, Fitsch EF, Maniatis T: Molecular Cloning: A Laboratory Manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY (1989).
Schmid D, Baici A, Gehring H, Christen P: Kinetics of molecular chaperone action. Science 263: 971–973 (1994).
Smith TF, Waterman MS: Identification of common molecular subsequences. J Mol Biol 147: 195–197 (1981).
Takahashi T, Komeda Y: Characterization of two genes encoding small heat shock proteins in Arabidopsis thaliana. Mol Gen Genet 219: 365–372 (1989).
Taylor MA, Davies HV, Smith SB, Abruzzese A, Gosling PG: Cold-induced changes in gene expression during dormancy breakage in seeds of Douglas fir (Pseudotsuga menziesii). J Plant Physiol 142: 120–123 (1993).
Tranbarger TJ, Misra S: The molecular characterization of a set of cDNAs differentially expressed during Douglas-fir germination and early seeding development. Physiol Plant 95: 456–464 (1995).
Tseng TS, Yeh KW, Yeh CH, Chang FC, Chen YM, Lin CY: Two rice (Oryza sativa) full-length cDNA encoding low molecular weight heat shock proteins. Plant Mol Biol 18: 963–965 (1992).
Verwoerd TC, Dekker BMM, Hoekema A: A small scale procedure for the rapid isolation of plant RNAs. Nucl Acids Res 17: 2362 (1989).
Vierling E, Sun A: Developmental expression of heat shock proteins in higher plants. In: Cherry JH (ed) Environmental Stress in Plants: Biochemical and Physiological Considerations. pp. 354–354. Springer-Verlag, New York (1989).
Vierling E: The role of heat shock proteins in plants. Annu Rev Plant Physiol Plant Mol Biol 42: 579–620 (1991).
Waters R, Lee GJ, Vierling E: Evolution, structure and function of the small heat shock proteins in plants. J Exp Bot (in press).
Weng J, Wang Z-F, Nguyen HT: Molecular cloning and sequence analysis of cDNAs encoding cytoplasmic low molecular weight heat shock proteins in hexaploid wheat. Plant Sci 92: 35–46 (1993).
Wiech H, Buchner J, Zimmermann R, Jakob U: Hsp90 chaperone protein folding in vitro. Nature 358: 169–170 (1992).
Wynn RM, Davie JR, Cox RP, Chuang DT: Molecular chaperones: heat shock proteins, foldases and match-makers. J Lab Clin Med 124: 31–36 (1994).
Zarsky V, Garrido D, Eller N, Tupy J, Vicente O, Schoffl F, Heberle-Bors E: The expression of a small heat shock gene is activated during induction of tobacco pollen embryogenesis by starvation. Plant Cell Environ 18: 139–147 (1995).
Zimmerman JL, Apuya N, Darwish K, O'Carroll C: Novel regulation of heat shock genes during carrot somatic embryo development. Plant Cell 1: 1137–1146 (1989).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kaukinen, K.H., Tranbarger, T.J. & Misra, S. Post-germination-induced and hormonally dependent expression of low-molecular-weight heat shock protein genes in Douglas fir. Plant Mol Biol 30, 1115–1128 (1996). https://doi.org/10.1007/BF00019546
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00019546