Abstract
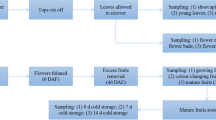
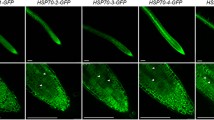
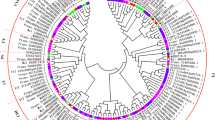
Eukaryotes express several cytoplasmic HSP70 genes, and their encoded proteins participate in diverse cellular processes. Three cDNAs encoding highly expressed cytoplasmic HSP70 homologues from Pisum sativum were cloned and characterized. They were designated PsHSP71.2, PsHSC71.0, and PsHSP70b. These HSP70 genes have different expression profiles in leaves: PsHSP71.2 is observed only in response to heat stress, PsHSC71.0 is present constitutively, and PsHSP70b is weakly constitutively expressed, but induced strongly in response to heat stress. In addition to being heat induced, the PsHSP71.2 mRNA is also expressed in zygotic, but not maternal organs of developing pea seeds, while PsHSC71.0 and PsHSP70b mRNAs are present in maternal and zygotic organs throughout seed development. Immunoblot analysis of parallel protein samples detects a 70 kDa polypeptide in all samples, and a 72 kDa polypeptide that corresponds to the PsHSP71.2 gene product is observed in cotyledons beginning at mid-maturation and in axes beginning between late maturation and desiccation. This polypeptide is not detected in the seed coat. The 72 kDa polypeptide remains abundant in both cotyledons and axes through germination, but declines substantially between 48 and 72 h after the onset of imbibition. Differential control of HSP70 expression during heat stress, seed maturation, and germination is consistent with the hypothesis that there are functional distinctions between cytoplasmic HSP70s.
Similar content being viewed by others
References
Beckmann RP, Mizzen LA, Welch WJ: Interaction of Hsp 70 with newly synthesized proteins: implications for protein folding and assembly. Science 248: 850–54 (1990).
Blond-Elguindi S, Cwirla SE, Dower WJ, Lipshutz RJ, Sprang SR, Sambrook JF, Gething MJH: Affinity panning of a library of peptides displayed on bacteriophages reveals the binding specificity of BiP. Cell 75, 717–728 (1993).
Boston RS, Fontes EBP, Shank BB, Wrobel RL: Increased expression of the maize immunoglobulin binding protein homolog b-70 in three zein regulatory mutants. Plant Cell 3: 497–505 (1991).
Bray EA: Drought-stress-induced polypeptide accumulation in tomato leaves. Plant Cell Environ 13: 531–538 (1990).
Chappell TG, Welch WJ, Schlossman DM, Palter KB, Schlesinger MJ, Rothman JE: Uncoating ATPase is a member of the 70 kilodalton family of stress proteins. Cell 45: 3–13 (1986).
Chen Q, Lauzon LM, DeRocher AE, Vierling E: Accumulation, stability, and localization of a major chloroplast heat-shock protein. J. Cell Biol. 110: 1873–1883 883 (1990).
Dang CV, Lee WMF: Nuclear and nucleolar targeting sequences of c-erb-A, c-myc, N-myc, p53, HSP70, and HIV tat proteins. J Biol Chem 264: 18019–18023 (1989).
Denecke J, Goldman MHS, Demolder J, Seurinck J, Botterman J: The tobacco luminal binding protein is encoded by a multigene family. Plant Cell 3: 1025–1035 (1991).
DeRocher AE, Helm KW, Lauzon LM, Vierling E: Expression of a conserved family of cytoplasmic low molecular weight heat shock proteins during heat-stress and recovery. Plant Physiol 96: 1038–1047 (1991).
DeRocher AE, Vierling E: Developmental control of small heat shock protein expression during pea seed maturation. Plant J 5: 93–102 (1994).
Devereux J, Haeberli P, Smithies O: A comprehensive set of sequence analysis programs for the VAX. Nuc Acids Res 12, 387–395 (1984).
Domoney C, Ellis N, Turner L, Casey R: A developmentally regulated early-embryogenesis protein in pea (Pisum sativum L.) is related to the heat-shock protein (HSP70) gene family. Planta 184: 350–355 (1991).
Duck N, McCormick S, Winter J: Heat shock protein hsp 70 cognate gene expression vegetative and reproductive organs of Lycopersicon esculentum. Proc Natl Acad Sci USA 86: 3674–3678 (1989).
Feinberg AP, Vogelstein B: A technique of radiolabeling DNA restrirtion endonuclease fragments to high specific activity. Anal Biochem 132: 6–13 (1983).
Flaherty KM, DeLuca-Flaherty C, McKay DB: Three-dimensional structure of the ATPase fragment of a 70K heat-shock cognate protein. Nature 346: 623–628 (1990).
Flynn GC, Pohl J, Flocco MT, Rothman JE: Peptidebinding specificity of the molecular chaperone BiP. Nature 353: 726–730 (1991).
Fontes EBP, Shank BB, Wrobel RL, Moose SP, OBrian GR, Wurtzel ET, Boston RS: Characterization of an immunoglobulin binding protein homolog in the maize floury-2 endosperm mutant. Plant Cell 3: 483–496 (1991).
Gao B, Biosca J, Craig EA, Greene LE, Eisenberg E: Uncoating of coated vesicles by yeast hsp 70 proteins. J Biol Chem 266: 19565–19571 (1991).
Gething MJ, Sambrook J: Protein folding in the cell. Nature 355: 33–35 (1992).
Ghosh S, Gepstein S, Heikkila JJ, Dumbroff EB: Use of a scanning densitometer or ELISA plate reader for measurement of nanogram amounts of protein in crude extracts from biological tissues. Anal Biochem 169: 227–233 (1988).
Green LAD, Liem RKL: β-internexin is a microtubule-associated protein identical to the 70-kDa heat-shock cognate protein and the clathrin uncoating ATPase. J Biol Chem 264: 15210–15215 (1989).
Greene LE, Eisenberg E: Dissociation of clathrin from coated vesicles by the uncoating ATPase. J Biol Chem 65: 6682–6687 (1990).
Guidon PT, Hightower LE: Purification and initial characterization of the 71-kilodalton rat heat-shock protein and its cognate as fatty acid binding proteins. Biochemistry 25: 3231–3239 (1986).
Hartl FU, Martin J, Neupert W: Protein folding in the cell: the role of molecular chaperones Hsp70 and Hsp60. Annu Rev Biophys Biomol Struct 21: 293–322 (1992).
Kelly WL, Georgopoulos C: Chaperones and protein folding. Curr Opin Cell Biol 4: 984–991 (1992).
Li QB, Anderson JV, Guy CL: A cDNA clone encoding a spinach 70-kilodalton heat-shock cognate. Plant Physiol 105: 45–58 (1994).
Lin TY, Duck NB, Winter J, Folk WR: Sequences of two hsc70 cDNAs from Lycopersicon esculentum. Plant Mol Biol 16: 47–78 (1991).
Lin X, Chern MS, Zimmerman JL: Cloning and characterization of a carrot hsp70 gene. Plant Mol Biol 17: 1245–1249 (1991).
Marshall JS, DeRocher AE, Keegstra K, Vierling E: Identification of heat shock protein hsp70 homologues in chloroplasts. Proc Natl Acad Sci USA 87: 374–378 (1990).
Marshall JS, Keegstra K: Isolation and characterization of a cDNA clone encoding the major Hsp 70 of the pea chloroplastic stroma. Plant Physiol 100: 1048–1054 (1992).
Milarski KL, Welch WJ, Morimoto RI: Cell cycle-dependent association of HSP70 with specific cellular proteins. J Cell Biol 108: 413–423 (1989).
Müller C, Igloi GL, Beck CF: Structure of a gene encoding heat-shock protein HSP70 from the unicellular alga Chlamydomonas reinhardtii. Gene 111: 165–173 (1992).
Nelson JR, Ziegelhoffer T, Nicolet C, Werner-Washburne M, Craig EA: The translation machinery and 70 kd heat shock protein cooperate in protein synthesis. Cell 71: 97–105 (1992).
Neumann D, zur Nieden U, Manteuffel R, Walter G, Scharff KD, Nover L: Intracellular localization of heat-shock proteins in tomato cell cultures. Eurt J Cell Biol 43: 71–81 (1987).
Nover L: Control of HSP synthesis. In: Nover L (ed) Heat Shock Response, pp. 237–279. CRC Press, Boca Raton, FL (1991).
Nover L, Scharf KD: Synthesis, modification and structural binding of heat-shock proteins in tomato cell cultures. Eur J Biochem 139: 303–313 (1984).
Perrey R, Wink M: Constitutive expression and molecular characterization of a cDNA clone encoding a partial HSP70 gene in cell suspension cultures of Lupinus polyphyllus. J Plant Physiol 137: 744–748 (1991).
Rippmann F, Taylor WR, Rothbard JB, Green NM: A hypothetical model for the peptide binding domain of hsp70 based on the peptide binding domain of HLA. EMBO J 10: 1053–1059 (1991).
Roberts JK, Key JL: Isolation and characterization of a soybean hsp70 gene. Plant Mol Biol 16: 671–683 (1991).
Rochester DE, Winter JA, Shah DM: The structure and expression of maize genes encoding the major heat shock protein, hsp70. EMBO J 5: 451–458 (1986).
Schuster AM, Davies E: Ribonucleic acid and protein metabolism in pea epicotyls. Plant Physiol 73: 809–816 (1983).
Shi Y, Thomas JO: The transport of proteins into the nucleus requires the 70-kilodalton heat shock protein or its cytosolic cognate. Mol Cell Biol 12: 2186–2192 (1992).
Stevenson MA, Calderwood SK: Members of the 70-kilodalton heat shock protein family contain a highly conserved calmodulin-binding domain. Mol Cell Biol 10: 1234–1238 (1990).
Velazquez JM, Lindquist S: hsp70: Nuclear concentration during environmental stress and cytoplasmic storage during recovery. Cell 36: 655–662 (1984).
Vierling E, Nagao RT, DeRocher AE, Harris LM: A heat shock protein localized to chloroplasts is a member of a eukaryotic superfamily of heat shock proteins. EMBO J 7: 575–581 (1988).
von Gromoff ED, Treier U, Beck CF: Three light inducible heat shock genes of Chlamydomonas reinhardtii. Mol Cell Biol 9: 3911–3918 (1989).
Wang C, Lin BL: The disappearance of an hsc70 species in mung bean seed during germination: purification and characterization of the protein. Plant Mol Biol 21: 317–329 (1993).
Watts FZ, Walters AJ, Moore AL: Characterization of PHSP1, a cDNA encoding a mitochondrial HSP70 from Pisum sativum. Plant Mol Biol 18: 23–32 (1992).
Welch WJ, Mizzen LA: Characterization of the thermotolerant cell. II Effects on the intracellular distribution of heat-shock protein 70. intermediate filaments and small nuclear ribonucleoprotein complexes. J Cell Biol 106: 1117–1130 (1988).
Wetmur JG: DNA probes: applications of the principles of nucleic acid hybridization. Crit Rev Biochemad Mol Biol 26: 227–259 (1991).
Winter J, Wright R, Duck N, Gasser C, Fraley R, Shsh D: The inhibition of petunia Hsp70 mRNA processing during CdCl2 stress. Mol Gen Genet 211: 315–319 (1988).
Wu CH, Caspar T, Browse J, Lindquist S, Somerville C: Characterization of an HSP70 cognate gene family in Arabidopsis. Plant Physiol 88: 731–740 (1988).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
DeRocher, A., Vierling, E. Cytoplasmic HSP70 homologues of pea: differential expression in vegetative and embryonic organs. Plant Mol Biol 27, 441–456 (1995). https://doi.org/10.1007/BF00019312
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00019312