Abstract
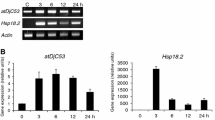
Mitochondria contain a nuclear-encoded heat shock protein, HSP60, which functions as a chaperonin in the post-translational assembly of multimeric proteins encoded by both nuclear and mitochondrial genes. We have isolated and sequenced full-length complementary DNAs coding for this mitochondrial chaperonin in Arabidopsis thaliana and Zea mays. Southern-blot analysis indicates the presence of a single hsp60 gene in the genome of A. thaliana. There is a high degree of homology at the predicted amino acid levels (43 to 60%) between plant HSP60s and their homologues in prokaryotes and other eukaryotes which indicates that these proteins must have similar evolutionarily conserved functions in all organisms. Northern- and western-blot analyses indicate that the expression of the hsp60 gene is developmentally regulated during seed germination. It is also heat-inducible. Developmental regulation of the (β-subunit) of F1-ATPase, an enzyme complex that is involved in the cyanide-sensitive mitochondrial electron transport system, indicates that imbibed embryos undergo rapid mitochondrial biogenesis through the early stages of germination. Based on the functional role of HSP60 in macromolecular assembly, these data collectively suggest that the presence of higher levels of HSP60 is necessary during active mitochondrial biogenesis, when the need for this protein is greatest in assisting the rapid assembly of the oligomeric protein structures.
Similar content being viewed by others
References
Barraclough R, Ellis RJ: Protein synthesis in chloroplasts. IX. Assembly of newly synthesized large subunits into ribulose bisphosphate carboxylase in isolated pea chloroplasts. Biochim Biophys Acta 608: 19–31 (1980).
Bienz M: Transient and developmental activation of heat-shock genes. Trends Biochem Sci 10: 157–161 (1985).
Blair GE, Ellis RJ: Protein synthesis in chloroplasts I: Light-driven synthesis of the large subunit of fraction I protein by isolated pea chloroplasts. Biochim Biophys Acta 319: 223–234 (1973).
Bochkareva ES, Lissin NM, Girshovich AS: Transient association of newly-synthesized unfolded proteins with the heat-shock Gro-EL protein. Nature 336: 254–257 (1988).
Burke TJ, Callis J, Vierstra RD: Characterization of a polyubiquitin gene from Arabidopsis thaliana. Mol Gen Genet 231: 435–443 (1988).
Cheng NY, Hartl F-U, Martin J, Pollack RA, Kalousek F, Neupert W, Hallberg EM, Hallberg RL, Horwich AL: Mitochondrial heat shock protein hsp60 is essential for assembly of proteins imported into yeast mitochondria. Nature 337: 620–625 (1989).
Chirico WJ, Waters MG, Blobel G: 70K heat shock proteins stimulate protein translocation into microsomes. Nature 332: 805–810 (1988).
Chitnis PR, Nelson N: Molecular cloning of the genes encoding two chaperone proteins of the cyanobacterium Synechocystis sp. pCC 6803. J Biol Chem 266: 58–65 (1991).
Day DA, Hanson JB: On methods for the isolation of mitochondria from etiolated corn shoots. Plant Sci Lett 11: 99–104 (1977).
Deshaies RJ, Koch BD, Werner-Washburne M, Craig EW, Schekman: A subfamily of proteins facilitates translocation of secretory and mitochondrial precursor polypeptides. Nature 332: 800–805 (1988).
Devereux J, Haeberli P, Smithies O: A comprehensive set of sequence programs for the VAX. Nucl Acids Res 12: 387–395 (1984).
Doyle JJ, Doyle JL: A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull 19: 11–15 (1987).
Ehrenshaft M, Brambl R: Respiration and mitochondrial biogenesis in germinating embryos of maize. Plant Physiol 93: 295–304 (1990).
Ellis RJ: Molecular chaperones: The plant connection. Science 250: 954–959 (1990).
Ellis RJ, Hemmingsen SM: Molecular chaperones: Proteins essential for the biogenesis of some macromolecular structures. Trends Biochem Sci 14: 339–342 (1989).
Georgopoulos CP, Hendrix RN, Casjens SR, Kaiser AD: Host participation in bacteriophage k head assembly. J Mol Biol 76: 45–60 (1973).
Goloubinoff P, Christeller JT, Gatenby AA, Lorimer GH: Reconstitution of active dimeric ribulose bisphosphate carboxylase from an unfolded state depends on two chaperonin proteins and Mg-ATP. Nature 342: 884–889 (1990).
Goloubinoff P, Gatenby AA, Lorimer GH: GroE heat-shock proteins promote assembly of foreign prokaryotic ribulose bisphosphate carboxylase oligomers in Escherichia coli. Nature 337: 44–47 (1989).
Haffner MH, Chen MB, Lane BG: Wheat embryo ribonucleotides XII. Formal characterization of terminal and penultimate residues at the 5′-ends of ‘capped” RNA from imbibing wheat embryos. Can J Biochem 54: 729–733 (1978).
Helm KW, Abernethy RH: Heat shock proteins and their mRNAs in dry and early imbibing embryos of wheat. Plant Physiol 93: 1626–1633 (1990).
Hemmingsen SM, Woolford C, van derVies SM, Tilly K, Dennis DT, Georgopoulos CP, Hendrix RW, Ellis RJ: Homologous plant and bacterial protein chaperone oligomeric assembly. Nature 333: 330–334 (1988).
Harlow E, Lane D (eds) Antibodies: A Laboratory Manual. Cold Spring Harbor Laboratory. Cold Spring Harbor, NY (1988).
Jindal S, Dudani AK, Singh B, Harley CB, Gupta RS: Primary structure of a human mitochondrial protein homologous to the bacterial and plant chaperonins and to the 65-kilodalton mycobacterial antigen. Mol Cell Biol 9: 2279–2283 (1989).
Johnson RB, Fearon K, Mason T, Jindal S: Cloning and characterization of the yeast chaperonin HSP60 gene. Gene 84: 295–302 (1989).
Kang P-J, Ostermann J, Shilling J, Neupert W, Craig EA, Pfanner N: Requirement for hsp70 in the mitochondrial matrix for translocation and folding of precursor proteins. Nature 348: 137–143 (1990).
Key JL, Czarnecka E, Gurley WB, Nagao RT: An analysis of physiological and molecular aspects of heat shock gene expression. In: Bruening G, Harada J, Kosuge T, Hollaender A (eds) Tailoring Genes for Crop Improvement, pp. 101–109. Plenum Publishing corporation (1987).
Kusukawa N, Yura T: Heat shock protein GroE of Escherichia coli: Key protective roles against thermal stress. Genes Devel 2: 874–882 (1988).
Levings CSIII, Brown GG: Molecular biology of plant mitochondria. Cell 56: 171–179 (1989).
Lissin NM, Venyanimov SYu, Girshovich AS: (Mg-ATP)-dependent self-assembly of molecular chaperone GroEL. Nature 348: 339–342 (1990).
Lubben TH, Donaldson GK, Vitanen PV, Gatenby AA: Several proteins imported into chloroplasts from stable complexes with the GroEL-related chloroplast molecular chaperone. Plant Cell 1: 1223–1230 (1989).
Lubben TH, Gatenby AA, Donaldson GK, Lorimer GH, Vitanen PV: Identification of a GroES-like chaperonin in mitochondria that facilitates protein folding. Proc Natl Acad Sci USA 87: 7683–7687 (1990).
Lundquist S, Craig EA: The heat shock proteins. Annu Rev Genet 22: 631–677 (1988).
Marshall JS, DeRocker AE, Keegstra K, Vierling E: Identification of heat shock protein hsp70 homologues in chloroplasts. Proc Natl Acad Sci USA 87: 374–378 (1990).
Martel R, Cloney LP, Pelcher LE, Hemmingsen SM: Unique composition of plastid chaperonin-60: a and β polypeptide-encoding genes are highly divergent. Gene 94: 181–187 (1990).
McMullin TW, Hallberg RL: A highly evolutionarily conserved mitochondrial protein is structurally related to the protein encoded by the E. coli groEL gene. Mol Cell Biol 8: 371–380 (1988).
Morohashi Y, Bewley JD: Development of mitochondrial activities in pea cotyledons during and following germination of the axis. Plant Physiol 51: 833–838 (1980).
Morohashi Y, Bewley JD, Yeung EC: Biogenesis of mitochondria in imbibed peanut cotyledons: Influence of the axis. J Exp Bot 32: 605–613 (1981).
Murray EE, Lotzer J, Eberle M: Codon usage in plant genes. Nucl Acids Res 17: 477–498 (1989).
Nagao RT, Kimpel JA, Vierling E, Key JL: The heat shock response: A comparative analysis. Oxford Sur Plant Mol Cell Biol 3: 384–438 (1986).
O'Farrell PH: High-resolution two-dimensional electrophoresis of proteins. J Biol Chem 250: 4007–4021 (1975).
Ostermann J, Horwich AL, Neupert W, Hartl F-U: Protein folding in mitochondria requires complex formation with hsp60 and ATP hydrolysis. Nature 341: 125–130 (1989).
Pelham HRB: Speculations on the functions of the major heat shock and glucose regulated proteins. Cell 46: 959–961 (1986).
Pelham HRB: Heat shock proteins: coming in from the cold. Nature 332: 776–777 (1988).
Prasad TK, Hack E, Hallberg RL: Function of the maize mitochondrial chaperonin hsp60: specific association between hsp60 and newly synthesized F1-ATPase alpha subunits. Mol Cell Biol 10: 3979–3986 (1990).
Prasad TK, Hallberg RL: Identification and metabolic characterization of the Zea mays mitochondrial homolog of the Escherichia coli GroEL protein. Plant Mol Biol 12: 609–618 (1989).
Reading DS, Hallberg RL, Myers AM: Characterization of the yeast hsp60 gene coding for a mitochondrial assembly factor. Nature 337: 655–659 (1989).
Rothman JE: Polypeptide chain binding proteins: catalysis of protein folding and related processes in cells. Cell 59: 591–601 (1989).
Roy H: Rubisco Assembly: a model system for studying the mechanism of chaperonin action. Plant Cell 1: 1035–1042 (1989).
Roy H, Bloom M, Milos P, Monroe M: Studies on the assembly of large subunits of ribulose bisphosphate carboxylase in isolated pea chloroplasts. J Cell Biol 94: 20–27 (1982)
Sambrook J, Fritsch EF, Maniatis T: Molecular Cloning: A Laboratory Manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY (1989).
Sanger F, Nicklen S, Coulson AC: DNA sequencing with chain terminating inhibitors. Proc Natl Acad Sci USA 74: 5463–5467 (1977).
Scherer PE, Krieg UC, Hwang ST, Vestweber D, Schatz G: A precursor protein partly translocated into yeast mitochondria is bound to a 70 kd mitochondrial stress protein. EMBO J 9: 4315–4322 (1990).
Skowyra D, Georgopoulos C, Zyliez M: The E. coli dnaK gene product, the hsp 70 homolog, can reactivate heatinactivated RNA polymerase in an ATP hydrolysis-dependent manner. Cell 62: 939–944 (1990).
Verner K, Schatz G: Protein translocation across membranes. Science 241: 1307–1313 (1988).
Viera J, Messing J: Production of single-stranded plasmid DNA. Meth Enzymol 153: 3–11 (1987).
Vierling E: The roles of heat shock proteins in plants. Annu Rev Plant Physiol Plant Mol Biol 42: 579–620 (1991).
Vierling E, Sun A: Developmental expression of heat shock proteins in higher plants. In: JCherry (ed) Environmental Stress in Plants: Biochemical and Physiological Mechanisms Associated with Environmental Stress tolerance in Plants, pp. 343–354. Springer-Verlag, New York (1988).
vonHeijne G: Mitochondrial targeting sequences may form amphiphilic helices. EMBO J 5: 1335–1342 (1986).
Voinikov VK, Rudikovskii AV: Association of heat shock proteins of corn with mitochondria in vivo and in vitro. Figiol Rastinii 35: 542–547 (1988).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Prasad, T.K., Stewart, C.R. cDNA clones encoding Arabidopsis thaliana and Zea mays mitochondrial chaperonin HSP60 and gene expression during seed germination and heat shock. Plant Mol Biol 18, 873–885 (1992). https://doi.org/10.1007/BF00019202
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00019202