Abstract
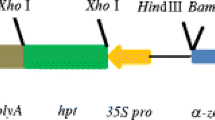
The 5′ upstream region of the rice storage protein type II glutelin gene was examined for its regulatory function in transgenic tobacco. Chimeric genes containing 5′ flanking regions of the glutelin gene transcriptionally fused to the β-glucuronidase (GUS) reporter gene were introduced into the tobacco genome by Agrobacterium tumefaciens-mediated gene transfer. The chimeric genes were expressed specifically in developing seeds, as opposed to leaves and stems, of the transgenic tobacco. Histochemical analysis revealed that the GUS activity was restricted to the endosperm tissue. A deletion series of the 5′ flanking region was created from position -1329 to -74 relative to the transcriptional initiation site and similarly examined in transgenic tobacco. Measurement of GUS activity of the seeds from the transgenic plants bearing the chimeric genes indicated that the region between positions -441 and -237 was required for the temporal and endosperm-specific expression of the GUS activity in tobacco. RNA analysis by northern blotting confirmed the importance of the -441 to -237 region. Addition of up to 888 bp to the -441 deletion resulted in little increase in GUS activity, although all constructs expressing the GUS gene showed a similar tissue and temporal regulation pattern.
Similar content being viewed by others
References
BevanM: Binary Agrobacterium vectors for plant transformation: Nucleic Acids Res 12: 8711–8721 (1984).
BradfordMM: A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 (1976).
BustonMM, GuiltinanMJ, JordanoJ, BegumD, KalkanFA, HallTC: Regulation of β-glucuronidase expression in transgenic tobacco plants by an A/T-rich, cis-acting sequence found upstream of a French bean α-phaseolin gene. Plant Cell 1: 839–853 (1989).
ChenZL, SchulerMA, BeachyRN: Functional analysis of regulatory elements in a plant embryo-specific gene. Proc Natl Acad Sci USA 83: 8460–8564 (1986).
ChenZL, PanNS, BeachyRN: A DNA sequence element that confers seed-specific enhancement of a constitutive promoter. EMBO J 7: 297–302 (1988).
ColotV, RobertLS, KavanaghTA, BevanMW, ThompsonRD: Localisation of sequences in wheat endosperm protein genes which confer tissue specific expression in tobacco. EMBO J 6: 3559–3564 (1987).
FeinbergAP, VogelsteinB: A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem 132: 6–13 (1983).
FordeBG, HeyworthA, PywellJ, KreisM: Nucleotide sequence of a B1 hordein gene and the identification of possible upstream regulatory elements in endosperm protein genes from barley, wheat and maize. Nucleic Acids Res 13: 7327–7339 (1985).
GoldbergRB, BarkerSJ, Perez-GrauL: Gene expression during plant embryogenesis. Cell 56: 149–160 (1989).
HenikoffS: Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing, Gene 28: 351–359 (1984).
HorschRB, FryJE, HoffmannNL, EichholtzD, RogersSG, FraleyRT: A simple and general method for transferring genes into plants. Science 227: 1229–1231 (1985).
JeffersonRA: Assaying chimeric genes in plants: The GUS gene fusion system. Plant Mol Biol Rep 5: 387–405 (1987).
JordanoJ, AlmogueraC, ThomasTL: A sunflower helianthinin gene upstream sequence ensemble contains an enhancer and sites of nuclear protein interaction. Plant Cell 1: 855–866 (1989).
LeisyDJ, HniloY, ZhaoY, OkitaTW: Expression of a rice glutelin promoter in transgenic tobacco. Plant Mol Biol 14: 41–50 (1990).
MaierUG, BrownJWS, ToloczykiC, FeixG: Binding of a nuclear factor to a consensus sequence in the 5′ flanking region of zein genes from maize. EMBO J 6: 17–22 (1987).
ManiatisT, FritschEF, SambrookJ: Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY (1982).
MarrisC, GalloisP, CopleyJ, KreisM: The 5′ flanking region of a barley B hordein gene controls tissue and developmental specific CAT expression in tobacco plants. Plant Mol Biol 10: 359–366 (1988).
MasumuraT, KidzuK, SugiyamaY, MitsukawaN, HibinoT, TanakaK, FujiiS: Nucleotide sequence of a cDNA encoding a major rice glutelin. Plant Mol Biol 12: 723–725 (1989).
OkitaTW, HwangYS, HniloJ, KimWT, AryanAP, LarsonR, KrishnanHB: Structure and expression of the rice glutelin multigene family. J Biol Chem 265: 12573–12581 (1989).
RobertLS, ThompsonRD, FlavellR: Tissue-specific expression of a wheat high molecular weight glutelin gene in transgenic tobacco. Plant Cell 1: 569–578 (1989).
SangerF, NicklenS, CoulsonAR: DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74: 5463–5467 (1977).
SchernthanerJP, MatzkeMA, MatzkeAJM: Endosperm-specific activity of a zein promoter in transgenic tobacco plants. EMBO J 7: 1249–1255 (1988).
ShirsatA, WilfordN, CroyR, BoulterD: Sequence responsible for the tissue specific promoter activity of a pea legumin gene in tobacco. Mol Gen Genet 215: 326–331 (1989).
TakaiwaF, KikuchiS, OonoK: A rice glutelin family — A major type of glutelin mRNAs can be divided into two classes. Mol Gen Genet 208: 15–22 (1987).
TakaiwaF, EbinumaH, KikuchiS, OonoK: Nucleotide sequence of a rice glutelin gene. FEBS Lett 221: 43–47 (1987).
TakaiwaF, KikuchiS, OonoK: The complete nucleotide sequence of new type cDNA coding for rice storage protein glutelin. Nucleic Acids Res 17: 3289 (1989).
YamagataH, TanakaK: The site of synthesis and accumulation of rice storage proteins. Plant Cell Physiol 27: 135–145 (1986).
ZhaoWM, GatehouseJA, BoulterD: The purification and partial amino acid sequence of a polypeptide from the rice glutelin fraction of rice grains: homology to the pea legumin: FEBS Lett 162: 96–102 (1983).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Takaiwa, F., Oono, K. & Kato, A. Analysis of the 5′ flanking region responsible for the endosperm-specific expression of a rice glutelin chimeric gene in transgenic tobacco. Plant Mol Biol 16, 49–58 (1991). https://doi.org/10.1007/BF00017916
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00017916