Abstract
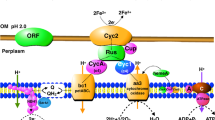
When ferrous iron and sulfur were supplied, cells of T. ferrooxidans in a well-aerated medium started growth by oxidizing ferrous iron. After ferrous iron depletion a lagphase followed before sulfur oxidation started. During sulfur oxidation at pH-values below 1.3 (±0,2) the ferrous iron concentration increased again, although the oxygen saturation of the medium amounted to more than 95%. The number of viable cells did not increase. Thus resting cells of T. ferrooxidans, which are oxidizing sulfur to maintain their proton balance, reduce ferric to ferrous iron. The ferrous iron-oxidizing system seemed to be inhibited at pH-values below 1.3. At a pH-value of 1.8 the ferrous iron was reoxidized at once. A scheme for the linkage of iron- and sulfur metabolism is discussed.
Similar content being viewed by others
References
Brierley CL (1982) Microbiological mining. Scientific American 247: 44–53
Brock TD & Gustafson J (1976) Ferric iron reduction by sulfur and iron oxidizing bacteria. Applied and Environmental Microbiology 32: 567–571
Anonymous (1984) Bestimmung von Eisen. In: Deutsche Einheitsverfahren zur Wasser-, Abwasser- und Schlammuntersuchung, El. Verlag Chemie, Weinheim
Dugan PR & Apel WA (1978) Microbiological desulfurization of coal (pp 223–250) In: Murr LA, Torma AE & Brierley JA(Eds) Metallurgical Applications of Bacterial Leaching and Related Microbiological Phenomena. Academic Press, New York
Eccleston M & Kelly DP (1978) Oxidation kinetics and chemostat growth kinetics of Thiobacillus ferrooxidans on tetrathionate and thiosulfate. Journal of Bacteriology 134: 718–727
Harrison AP Jr (1981) Acidophilium cryptum gen. nov., sp. nov., heterotrophic bacterium from acidic mineral environments. International Journal of Systematic Bacteriology 31: 327–332
Kelly DP (1982) Biochemistry of the chemolithotrophic oxidation of inorganic sulphur. Philosophic Transactions of the Royal Society, London Series B. 298: 499–528
Kino K & Usami S (1982) Biological reduction of ferric iron by iron and sulfur oxidizing bacteria. Agricultural Biology and Biochemistry 46: 803–805
Kulpa CF Mjoli N & Roskey MT (1986) Comparison of iron and sulfur oxidation in Thiobacillus ferroxidans: Inhibition of iron oxidation by growth on sulfur. In: Ehrlich HL & Holmes DS(Eds) Workshop on Biotechnology for the Mining, Metal-refining and Fossil Fuel Processing Industries. Biotechnology and Bioengineering Symposium No. 16, Interscience Publication. J. Wiley, New York
Lawrence RW & Gunn JD (1985) Biological preoxidation of a pyrite gold concentrate. In: Spisak JK & Jergensen GV(Eds) (pp 13–17) Frontier Technology in Mineral Processing. AIME, New York, NY
Lundgren DG, Boucheron J & Mahony W (1983) Geomicrobiology of iron: mechanisms of ferric iron reduction, In: Rossi G & Torma AE(Eds) Recent Progress in Biohydrometallurgy (pp 55–69) Associazione Mineraria Sarda, Italy
Mackintosh, M.W., 1978. Nitrogen fixation by Thiobacillus ferrooxidans. Journal of General Microbiology 105: 215–218
Marsh, R.M. and Norris P.R., 1983. Mineral sulphide oxidation by moderately thermophilic acidophilic bacteria. Biotechnology Letters 5: 585–590
Murr, L.E. and A.P. Metha, 1982. Coal desulfurization by leaching involving acidophilic and thermophilic microorganisms. Biotechnology Bioengineering 24: 743–748
Myerson, A.S., 1981. Oxygen mass transfer requirements during the growth of Thiobacillus ferrooxidans on iron pyrite. Biotechnology Bioengineering 23: 1413–1416
Silver, M. and D.G. Lundgren, 1968. Sulfur oxidizing enzyme of Thiobacillus ferrooxidans. Canadian Journal of Biochemistry 46: 457–461
Sugio, T., C. Domatsu, O. Munakata, T. Tano, and K. Imai, 1985. Role of a ferric iron reducing system in sulfur oxidation of Thiobacillus ferrooxidans. Applied and Environmental Microbiology 49: 1401–1406
Sundermeyer-Klinger, H., W. Meyer, B. Warninghoff, and E. Bock, 1984. Membrane-bound nitrite oxidoreductase of Nitrobacter: evidence for a nitrate reductase system. Archives of Microbiology 140: 153–158
Suzuki, I., 1965. Oxidation of elemental sulfur by an enzyme system of Thiobacillus ferrooxidans. Biochimica et Biophysica Acta 104: 359–371
Tuovinen, O.H. and D.P. Kelly, 1974. Studies on the growth of Thiobacillus ferrooxidans. Archives of Microbiology 95: 165–180
Vian M, Creo C, Dalmastri C, Gionni A, Palazzolo P & Levi G (1986) Thiobacillus ferrooxidans selection in continuous culture. In: Lawrence RW, Branion RMR & Ebner HG(Eds) Fundamental and Applied Biohydrometallurgy, (pp 395–401) Elsevier, Netherlands
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sand, W. Ferric iron reduction by Thiobacillus ferrooxidans at extremely low pH-values. Biogeochemistry 7, 195–201 (1989). https://doi.org/10.1007/BF00004217
Issue Date:
DOI: https://doi.org/10.1007/BF00004217