Abstract
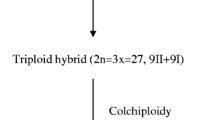
The genus Medicago comprises more than 60 species. Lesins and Gilles (1972) report 62 species, but some of them are interfertile and would be better defined as varieties. The native area of the genus is the Mediterranean Basin and the Middle East, but today it is dispersed all over the world. The genus can be divided into annuals and perennials, diploid and polyploid species. The basic chromosome number (x) is 7 or 8 so that the diploid species have 14 or 16 chromosomes. There are some autotetraploid species (2n = 32), and two species are hexaploid (2n = 48). Phylogenetic investigations have been carried out with chromosomal analysis (Mariani 1968) and more recently with isoenzymatic (Mariani et al. 1978; Quiros 1983) and restriction fragment length (Johnson and Palmer 1986) polymorphism.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
App BA, Manglitz GR (1972) Insects and related pests. In: Hanson CH (ed) Alfalfa science and technology. Am Soc Agron, Madison, WI, pp 527–554
Arcioni S, Davey MR, Dos Santos AVP, Cocking EC (1982) Somatic embryogenesis in tissues from mesophyll and cell suspension protoplasts of Medicago coerulea and M. glutinosa. Z Pflanzenphysiol 106: 105–110
Arcioni S, Pezzotti M, Damiani F (1987) In vitro selection of alfalfa plants resistant to Fusarium oxysporum f. spp. medicaginis. Theor Appl Genet 74: 700–705
Arcioni S, Pupilli F, Pezzotti M, Falistocco E, Damiani F (1987) Interspecific hybrid lines of M. sativa and M. arborea by protoplast electrofusion. In: Puite KJ, Dons JJM, Huizing HJ, Kool AJ, Koorneef M, Krens FA (eds) Progress in plant protoplast research. Kluwer, Dordrecht Boston London, pp 259–260
Arcioni S, Pezzotti M, Damiani F, Pupilli F (1988) Plant regeneration study and somaclonal variation in Medicago sativa. In: Rep 31st North American alfalfa improvement conference, June 19–23, 1988, Greenbelt, Maryland, p 83
Atanassov A, Brown DCW (1984) Plant regeneration from suspension culture and mesophyll protoplasts of Medicago sativa L. Plant Cell Tissue Organ Cult 3: 149–162
Baertlein DA, McDaniel RG (1987) Molecular divergence of alfalfa somaclones. Theor Appl Genet 73: 575–580
Bajaj YPS (1979 a) Technology and prospects of cryopreservation of germplasm. Euphytica 28:267–285
Bajaj YPS (1979b) Establishment of germplasm banks through freeze-storage of plant tissue culture and their implications in agriculture. In: Sharp WR, Larsen PO, Paddock EF, Raghvan V (eds) Plant cell and tissue culture: principles and applications. Ohio State Univ Press, Columbus, pp 745–774
Bajaj YPS (1983) In vitro production of haploids. In: Ammirato PV, Evans DA, Sharp WR, Yamada Y (eds) Handbook of plant cell culture 1. McMillan, New York, pp 228–287
Bajaj YPS (ed) (1989) Biotechnology in agriculture and forestry. 9 protoplasts and genetic engineering II. Springer, Berlin Heidelberg New York Tokyo
Bauchan GR (1987) Embryo culture of Medicago scutellata and M. sativa. Plant Cell Tissue Organ Cult 10: 21–29
Bequerel PM (1950) La via latente des graines aux confins du zéro absolute. CR Hebd Sci 231: 1274
Bingham ET (1969) Haploids of cultivated alfalfa (Medicago sativa L.). Nature (Lond) 22: 865–866
Bingham ET (1971) Isolation of haploids of tetraploid alfalfa. Crop Sci 11: 433–435
Bingham ET (1979) Maximizing heterozygosity in autopolyploids. In: Lewis WH (ed) Polyploidy, biological relevance. Plenum Press, New York, pp 471–489
Bingham ET, McCoy TJ (1986) Somaclonal variation in alfalfa. In: Janick J (ed) Plant Breeding Reviews, vol 4. AVI, Westport, Connecticut, pp 123–152
Bingham ET, Hurley LV, Kaatz DM, Saunders JW (1975) Breeding alfalfa which regenerates from callus tissue in culture. Crop Sci 15: 719–721
Binarova P, Novak FJ (1984) Regulation and somatic embryo development in cell culture of alfalfa (Medicago sativa L.). In: Novak FJ, Havel L, Dolezel J (eds) Plant tissue and cell culture application to crop improvement. Proc Int Symp Olomouc, 24–29 September 1984. Czech Acad Sci Prague, pp 139–141
Binarova P, Dolezel J (1988) Alfalfa embryogenic cell suspension culture: growth and ploidy level stability. J Plant Physiol 133: 561–566
Blaydes DF (1966) Interaction of kinetin and various inhibitors in the growth of soybean tissue. Physiol Plant 19: 748–753
Bocsa I (1981) Amélioration du rendement en matière sèche chez la luzerne. In: Bosca I (ed) Proc EUCARPIA Fodder Crop Section. July 1–3, 1980, Agricultural Research Institute, Kompolt, Hungary, pp 19–28
Bolton JL, Goplen BP, Baezinger H (1972) World distribution and historical developments. In: Hanson CM (ed) Alfalfa science and technology. Am Soc Agron, Madison, WI, pp 1–34
Brown DCW, Atanassov A (1985) Role of genetic background in somatic embryogenesis in Medicago. Plant Cell Tissue Organ Cult 4: 111–122
Busbice JH, Wilsie CP (1966) Inbreeding depression and heterosis in autotetraploids with application to Medicago sativa L. Euphytica 15: 52–57
Busse WF (1927) Effects of low temperatures on germination of impermeable seeds. Bot Gaz 89: 169
Byrne MC, McDonnell RE, Wright MS, Carnes MG (1987) Strain and cultivar specificity in the Agrobacterium-soybem interaction. Plant Cell Tissue Organ Cult 8: 3–15
Chen THH, Marowitch J (1987) Screening of Medicago falcata germplasm for in vitro regeneration. J Plant Physiol 128: 271–277
Chen THH, Marowitch J, Thompson BG (1987) Genotypic effects on somatic embryogenesis and plant regeneration from callus cultures of alfalfa. Plant Cell Tissue Organ Cult 8: 73–81
Cheyne VA, Dale PJ (1980) Shoot tip culture in forage legumes. Plant Sci Lett 19: 303–309
Costantino P, Spano L, Pomponi M, Benvenuto E, Ancora G (1984) The T-DNA of Agrobacterium rhizogenes is transmitted through meiosis to the progeny of hairy root plants. J Mol Appl Genet 2: 465–470
Crouch ML (1982) Non-zygotic embryos of Brassica napus L. contain embryo specific storage proteins. Planta 156: 520–524
Croughan TP, Stavarek SJ, Rains DW (1978) Selection of a NaCl tolerant line of cultured alfalfa cells. Crop Sci 18: 959–963
Damiani F, Pezzotti M, Arcioni S (1988) Electric field mediated fusion of protoplasts of Medicago sativa L. and Medicago arborea L. J Plant Physiol 132: 474–479
Davis PA (1988) Cell culture of forage legumes for the production of nutritionally improved, bloat-safe lucerne. Ph D Thesis (submitted to the Australian National University)
Deak M, Kiss GB, Koncs C, Dudits D (1986) Transformation of Medicago by Agrobacterium-medizted gene transfer. Plant Cell Rep 5: 97–100
Demarly Y (1963) Genétique de tetraploides et amélioration des plantes. Ann Amelior Plant 13: 307–400
D’Hont A, Quétier F, Teoule E, Dattèe Y (1987) Mitochondrial and chloroplast DNA analysis of interspecific somatic hybrids of a Leguminosae: Medicago (alfalfa). Plant Science 53: 237–242
Dijak M, Brown DCW (1987) Patterns of direct and indirect embryogenesis from mesophyll protoplasts of Medicago sativa. Plant Cell Tissue Organ Cult 9: 121–130
Dos Santos AVP, Outka DE, Cocking EC, Davey MR (1980) Organogenesis and somatic embryogenesis in tissue derived from leaf protoplast and leaf expiants of Medicago sativa. Z Pflanzenphysiol 99: 261–270
Dos Santos AVP, Cutter EG, Davey MR (1983) Origin and development of somatic embryos in Medicago sativa L. (alfalfa). Protoplasma 117: 107–115
Duke JA (ed) (1981) Handbook of legumes of world economic importance. Plenum Press, New York
Dunbier MW, Bingham ET (1975) Maximum heterozygosity in alfalfa: results using haploid-derived autotetraploids. Crop Sci 15: 527–531
Elgin JH Jr, McMurtrey JE III, Schaeffer GW (1977) Attempted interspecific hybrydization of Medicago scutellata and M. sativa. Agron Abstr 69: 54
Finkle BJ, Zuval ME, Ulrich JM (1985) Cryoprotective compounds in the viable freezing of plant tissues. In: Kartha KK (ed) Cryopreservation of plant cells and organs. CRC, Boca Raton, Florida, pp 75–113
Frearson EM, Power JB, Cocking EC (1973) Isolation, culture and regeneration of Petunia leaf protoplasts. Dev Biol 33: 130–137
Fridriksson S, Bolton JL (1963 a) Development of the embryo of Medicago sativa L. after normal fertilization and after pollination by other species of Medicago. Can J Bot 41–33
Fridriksson S, Bolton JL (1963 b) Preliminary report on the culture of alfalfa embryos. Can J Bot 4: 439–440
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension culture of soybean root cells. Exp Cell Res 50: 151–158
Gilmour DM, Davey MR, Cocking EC (1986) Somatic hybridization in Medicago. In: 6th Int Congr plant tissue and cell cult, Abstr, Aug 3–8, Univ Minnesota, Minneapolis, p 435
Gilmour DM, Davey MR, Cocking EC (1987 a) Isolation and culture of heterokaryons following fusion of protoplasts from sexually compatible and sexually incompatible Medicago species. Plant Sci 53: 263–270
Gilmour DM, Davey MR, Cocking EC (1987 b) Plant regeneration from cotyledon protoplasts of wild Medicago species. Plant Sci 48: 107–112
Gilmour DM, Davey MR, Cocking EC, Pental D (1987 c) Culture of low numbers of forage legume protoplasts in membrane chambers. J Plant Physiol 126: 457–465
Gilmour DM, Golds TJ, Davey MR (1988) Medicago protoplasts: isolation, fusion, culture and plant regeneration. In: Bajaj YPS (ed) Biotechnology in agriculture and forestry. 8 protoplasts and genetic engineering I. Springer, Berlin Heidelberg New York Tokyo
Graham JK, Kritlow KW, Faulkner LR (1972) Disease. In: Hanson CH (ed) Alfalfa science and technology. Am Soc Agron, Madison, WI, pp 497–526
Graham JH, Frosheiser FI, Stuteville DL, Erwin DC (1979) A compendium of alfalfa diseases. Am Phytopathol Soc, St Paul, Minnesota
Groose RE, Bingham ET (1984) Variation in plants regenerated from tissue culture of tetraploid alfalfa heterozygous for several traits. Crop Sci 24: 655–658
Hanson CH (1961) Longevity of pollen and ovaries of alfalfa. Crop Sci 1: 114–116
Hanson CH, Campbell TA (1972) Vacuum-dried pollen of alfalfa ( Medicago sativa L.) viable after eleven years. Crop Sci 12: 874
Hartman CL, McCoy TJ, Knous TR (1984) Selection of alfalfa (Medicago sativa) cell lines and regeneration of plants resistant to the toxin (s) produced by Fusarium oxysporum f. spp. medicaginis. Plant Sci Lett 34: 183–194
Hlasmikova A (1977) Androgenesis in vitro evaluated from the aspect of genetics. Z Pflanzenzuecht 78: 44–56
Hooykaas PJJ, Schilperoort RA (1984) The molecular genetics of crown-gall tumorigenesis. Adv Genet 22: 209–283
Hougas RW, Peloquin SJ (1958) The potential of potato haploids in breeding and genetic research. Am Pot J 35:701 –707
Indiogine SEP, Mita G, Terzi M (1986) Isolation of variants resistant to sodium chloride and methyl- ammonium through somaclonal variation. In: 6th Int Congr plant tissue and cell culture, Abstr, Aug 3–8, Univ Minnesota, Minneapolis, p 377
Johnson LB, Palmer JD (1986) Some phylogenetic relationships within the genus Medicago as determined by chloroplast DNA restriction mapping. Communication at Joint Meeting of 30th NAAIC, July 27–31, 1986, St Paul, Minnesota
Johnson LB, Stuteville DL, Higgins RK, Skinner DZ (1981) Regeneration of alfalfa plants from protoplasts of selected Regen S clones. Plant Sci Lett 20: 297–304
Johnson LB, Stuteville DL, Higgins RK, Douglas HL (1982) Pectolyase Y-23 for isolating mesophyll protoplasts from several Medicago species. Plant Sci Lett 26: 133–137
Johnson LB, Stuteville DL, Schlarbaum SE, Skinner DZ (1984 a) Variation in phenotype and chromosome number in alfalfa protoclones regenerated from nonmutagenized calli. Crop Sci 24:948 –952
Johnson LB, Rose RJ, Schlarbaum SE, Stuteville PL (1984b) Effects of protocloning in “Regen S” alfalfa on somatic chromosomes and on mitochondrial and chloroplast DNA. Rep 29th Alfalfa Improv Conf Lethridge, Alberta, Canada, p 74
Kao KN (1977) Chromosomal behaviour in somatic hybrids of soybean Nicotiana glauca. Mol Gen Genet 150: 225–230
Kao KN, Michayluk MR (1975) Nutritional requirement for growth of Vicia hajastana cells and protoplasts at very low population density in liquid media. Planta 126: 105–110
Kao KN, Michayluk MR (1980) Plant regeneration from mesophyll protoplasts of alfalfa. Z Pflanzenphysiol 96: 135–141
Kao KN, Michayluk MR (1981) Embryoid formation in alfalfa cell suspension cultures from different plants. In Vitro 17: 645–648
Kao KN, Saleem M (1986) Improved fusion of mesophyll and cotyledon protoplasts with PEG and high pH-Ca++ solution. J Plant Physiol 122: 217–225
Kao KN, Wetter LR (1977) Advances in techniques of plant protoplast fusion and culture of heterokaryocytes. In: Brinkley BR, Porte K (eds) International cell biology. Rockefeller Univ Press, New York, p 216
Kasha KJ (1974) Haploids in higher plants: advances and potential. Univ Press, Guelph
Larkin PJ, Scowcroft WR (1981) Somaclonal variation: a novel source of variability from cell cultures for plant improvement. Theor Appl Genet 60: 197–214
Latunde-Dada AO, Lucas JA (1983) Somaclonal variation and reaction to Verticillium wilt in Medicago sativa L. plants regenerated from protoplasts. Plant Sci Lett 32: 205–211
Lesins K (1961) Mode of fertilization in relation to breeding methods in alfalfa. Z Pflanzenzuecht 43: 31–54
Lesins K, Gillies CB (1972) Taxonomy and cytogenetics of Medicago. In: Hanson CH (ed) Alfalfa science and technology. Am Soc Agron, Madison, WI, pp 53–86
Linsmaier EM, Skoog F (1965) Organic growth factor requirements of tobacco tissue cultures. Physiol Plant 18: 100–127
Lipman CB, Lewis GN (1934) Tolerance of liquid-air temperatures by seeds of higher plants for sixty days. Plant Physiol 9: 392
Lu DJ, Pental D, Cocking EC (1982) Plant regeneration for seedling cotyledon protoplasts. Z Pflanzenphysiol 107: 59–63
Lubenec PA (1967) Utilizing wild perennial species. Proc All Univ Lenin Acad Agric Sci 5: 19–22
Lucretti S, Dolezel J, Spano M, Moretti F (1986) Legume protoplast culture and conditions for fluorescent labelling and cell sorting. 6th Int Congr plant tissue and cell cult, Abstr, Aug 3–8, Univ Minnesota, Minneapolis, p 452
Lupotto E (1983) Propagation of an embryogenic culture of Medicago sativa L. Z Pflanzenphysiol 111: 95–104
Lupotto E (1986) The use of single somatic embryo culture in propagating and regenerating lucerne CMedicago sativa L.). Ann Bot 57: 19–24
Mariani A (1968) Impiego dell’incrocio interspecifico nel miglioramento genetico dell’erba medica. Genet Agr 22: 35–51
Mariani A, Arcioni S, Veronesi F (1978) Cytological analysis and electrophoretic patterns of seed proteins in Medicago sativa, Medicago glutinosa and their hybrids. Genet Agr 32: 21–39
Mariotti D, Arcioni S, Pezzotti M (1984 a) Regeneration of Medicago arborea L. plants from tissue and protoplasts culture of different organ origin. Plant Sci Lett 37: 149–156
Mariotti D, Davey MR, Draper J, Freeman JP, Cocking EC (1984 b) Crown-gall tumorigenesis in the forage legume Medicago sativa L. Plant Cell Physiol 25: 473–482
McCoy TJ (1985) Interspecific hybridization of Medicago sativa L. and M. rupestris M.B. using ovule-embryo culture. Can J Genet Cytol 27: 238–245
McCoy TJ, Bingham ET (1977) Regeneration of diploid alfalfa plants from cells grown in suspension culture. Plant Sci Lett 10: 59–66
McCoy TJ, Smith LY (1984) Uneven ploidy levels and a reproductive mutant required for interspecific hybridization of Medicago sativa L. and Medicago dzhawakhetica Bordz. Can J Genet Cytol 26: 511–518
McCoy TJ, Smith LY (1986) Interspecific hybridization of perennial Medicago species using ovule-embryo culture. Theor Appl Genet 71: 772–783
Meijer EGM, Brown DCW (1985) Screening of diploid Medicago sativa germplasm for somatic embryogenesis. Plant Cell Rep 4: 285–288
Meijer EGM, Brown DCW (1987) Role of exogenous reduced nitrogen and sucrose in rapid high frequency somatic embryogenesis in Medicago sativa. Plant Cell Tissue Organ Cult 10: 11–19
Meins F Jr (1983) Heritable variation in plant cell culture. Annu Rev Plant Physiol 34: 327–346
Melchers G, Labib G (1970) Die Bedeutung haploider höherer Pflanzen für Pflanzenphysiologie und Pflanzenzüchtung. Durch Antheres Kultur erzeugte Haploide, ein neuer Durchbruch für die Pflanzenzüchtung. Ber Dtsch Bot Ges 83: 129–150
Mezentsen AV (1981) Mass regeneration of plants from the cells and protoplasts of alfalfa. Dokl Vaskuil 4: 22–23
Michaud E, Leman WF, Rumbaugh MD (1987) World distribution and historical development. In: American Society of Agronomy (ed) Alfalfa and alfalfa improvement (in preparation)
Mitten DH, Sato SJ, Skokut TA (1984) In vitro regenerative potential of alfalfa germplasm sources. Crop Sci 24: 943–945
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15: 473–497
Nagarajan P, McKenzie JS, Walton PD (1986) Embryogenesis and plant regeneration of Medicago spp. in tissue culture. Plant Cell Rep 5: 77–80
Nagarajan P, Walton PD (1987) A comparison of somatic chromosomal instability in tissue culture regenerants from Medicago media Pers. Plant Cell Rep 6: 109–113
Nagata T, Ishii S (1979) A rapid method for isolation of mesophyll protoplasts. Can J Bot 57: 1820–1823
Noväk FJ, Konecna D (1982) Somatic embryogenesis in callus and cell suspension cultures of alfalfa CMedicago sativa L.). Z Pflanzenphysiol 105: 279–284
Noväk FJ, Hermelim T, Daskav S, Nesticky M (1986) In vitro mutagenesis in maize. In: Horn W, Jensen CJ, Odenbach W, Schieder O (eds) Genetic manipulation in plant breeding. De Gruyter, Berlin, pp 563–576
Oldemeyer RD (1956) Interspecific hybridization in Medicago. Agron J 48: 584–585
Orr W, Singh J, Brown DCW (1985) Induction of freezing tolerance in alfalfa cell suspension cultures. Plant Cell Rep 4: 15–18
Orton TJ (1984) Somaclonal variation: theoretical and practical consideration. In: Gustafson JP (ed) Gene manipulations in plant improvement. Plenum Press, New York, pp 427–468
Peloquin SJ, Hougas RW (1958) Fertility in two haploids of Solanum tuberosum. Science 128: 1340–1341
Pezzotti M, Arcioni S, Mariotti D (1984) Plant regeneraton from mesophyll, root and cell suspension protoplasts of Medicago sativa cv. Adriana. Genet Agr 38: 195–208
Pezzotti M, Pupilli F, Damiani F, Arcioni S (1988) Genetic transformation of M. sativa. Agr 42: 477
Phillips GC (1983) Screening alfalfas adapted to the south western United States for regenerator genotypes. In Vitro 19 (3): 265
Phillips GC, Collins GB (1979) In vitro tissue culture of selected legumes and plant regeneration from callus cultures of red clover. Crop Sci 19: 59–64
Quiros CF (1983) Alfalfa, Lucerne (Medicago sativa L.). In: Tanksley SD, Orton TJ (eds) Isozymes in plant genetics and breeding, Part B. Elsevier, Amsterdam, pp 253–294
Raynal G (1986) Les maladies de la luzerne en Europe. In: Guy P, Massenot M (eds) Compte-rendudes groupes de travail Medicago sativa. EUCARPIA, INRA, Paris, pp 7–14
Reich TJ, Iyer VN, Miki BL (1986) Efficient transformation of alfalfa protoplasts by the intranuclear microinjection of Ti plasmid. Bio/technology 4: 1001–1004
Reisch B, Bingham ET (1980) The genetic control of bud formation from callus cultures of diploid alfalfa. Plant Sci Lett 20: 71–77
Reisch B, Bingham ET (1981) Plants from ethionine-resistant alfalfa tissue cultures: variation in growth and morphological characteristics. Crop Sci 21: 783–788
Reisch B, Duke SH, Bingham ET (1981) Selection and characterization of ethionine-resistant alfalfa ( Medicago sativa L.) cell lines. Theor Appl Genet 59: 89–94
Rose RJ, Johnson LB, Kemble RJ (1984) Restriction endonuclease studies on the chloroplast and mitochondrial DNA, of alfalfa ( Medicago sativa L.) protoclones. Curr Top Plant Biochem Physiol 3: 178
Sakai A, Noshiro M (1975) Some factors contributing to the survival of crop seeds cooled to the temperature of liquid nitrogen. In: Frankel OH, Hawkes JG (eds) International Biology Program: Crop genetic resources for today and tomorrow. Cambridge Univ Press, Cambridge, pp 317–326
Sangduen N, Kreitner GL, Sorensen EL (1983) Light and electron microscopy of embryo development in an annual x perennial Medicago species cross. Can J Bot 61: 1241–1257
Saunders JW, Bingham ET (1972) Production of alfalfa plants from callus tissue. Crop Sci 12: 804–806
Saunders JW, Bingham ET (1975) Growth regulator effects on bud initiation in callus cultures of Medicago sativa. Am J Bot 62: 850–855
Schenk RU, Hildebrandt AC (1972) Medium and techniques for induction and growth of monocoty-ledonous and dicotyledonous plant cell cultures. Can J Bot 50: 199–204
Shahin EA, Spielmann A, Sukhapinda K, Simpson RB, Yashar M (1986) Transformation of cultivated alfalfa using disarmed Agrobacterium tumefaciens. Crop Sci 26: 1235–1239
Smith MK, McComb JA (1983) Selection for NaCl tolerance in cell cultures of Medicago sativa and recovery of plants from a NaCl-tolerant cell line. Plant Cell Rep 2: 126–128
Smith SE, Bingham ET, Fulton RW (1986) Transmission of chlorophyll deficiencies provides evidence for biparental inheritance of plastids in Medicago sativa. Heredity 77: 35–39
Somaroo BH, Witcombe JR (1982) The evaluation and utilization of annual medics at ICARDA. In: Hayward MD (ed) The utilization of genetic resources in fodder crop breeding. Rep EUCARPIA Fodder Crops Section, Aberystwyth, Wales, UK, 13–16 Sept 1982, pp 198–207
Spano L, Mariotti D, Pezzotti M, Damiani F, Arcioni S (1987) Hairy root transformation in alfalfa (Medicago sativa L.). Theor Appl Genet 73:523–530
Spano L, Mariotti D, Pezzotti M, Damiani F, Arcioni S (1987) Hairy root transformation in alfalfa ( Medicago sativa L. ). Theor Appl Genet 73: 523–530
Stavarek SJ, Croughan TP, Rains DW (1980) Regeneration of plants from long-term cultures of alfalfa cells. Plant Sci Lett 19: 2253–2261
Strickland GS, Nichol JW, Stuart DA (1987) Effect of carbohydrate source on somatic embryogenesis. Plant Sci 48: 113–121
Stuart DA, Strickland SG ( 1984 a) Somatic embryogenesis from cell cultures of Medicago sativa L. The role of the amino acid addition to the regeneration medium. Plant Sci Lett 34: 165–174
Stuart DA, Strickland SG (1984b) Somatic embryogenesis from cell cultures of Medicago sativa L.I. The interaction of amino acids with ammonium. Plant Sci Lett 34: 175–181
Stuart DA, Nelsen J, McCall CM, Strickland SG, Walker KA ( 1985 a) Physiology of the development of somatic embryos in cell cultures of alfalfa and celery. In: Zaitlin M, Day P, Hollaender A (eds) Biotechnology in plant science: relevance to agriculture in the eighties. Academic Press, New York, P 35
Stuart DA, Nelsen J, Strickland SG, Nichol JW ( 1985 b) Factors affecting developmental processes in alfalfa cell cultures. In: Proc Tennessee Symp on Propagation of Higher Plants. Knoxville, p 373
Stuart DA, Nelsen J (1988) Isolation and characterization of alfalfa 7S and 11S seed storage proteins. J Plant Physiol 132: 129–133
Stuart DA, Nelsen J, Nichol JW (1988) Expression of 7S and 1 IS alfalfa seed storage proteins in somatic embryos. J Plant Physiol 132: 134–139
Sukhapinda K, Spivey R, Shahin EA (1987) Ri-plasmid as a helper for introducing vector DNA into alfalfa plants. Plant Mol Biol 8: 209–216
Tanner GY, Moore AE, Arcioni S, Larkin PJ (1988) Initiation of non-physiological division in cultured lucerne microspores. In: International Congress on genetic manipulation in plant breeding; organized by EUCARPIA, 11–12 Sept, Helsingor, Denmark, p 74
Teoule E (1983) Hybridization somatique entre Medicago sativa L. et Medicago falcata L. CR Acad Sci Paris 297: 13–16
Teoule E, Dattee Y (1987) Recherche d’une méthode fiable de culture de protoplastes, d’hybridation somatique et de régénération chez Medicago. Agronomie 7: 575–584
Tepfer D (1984) Transformation of several species of higher plants by Agrobacterium rhizogenes: sexual transmission of the transformed genotype and phenotype. Cell 37: 955–967
Uchimiya H, Murashige T (1974) Evaluation of parameters in the isolation of viable protoplasts from cultured tobacco cells. Plant Physiol 54: 936–944
Walker KA, Sato S J (1981) Morphogenesis in callus tissues of Medicago sativa. The role of ammonium ion in somatic embryogenesis. Plant Cell Tissue Organ Cult 1: 109–121
Walker KA, Yu PC, Sato SJ, Jaworski EG (1978) The hormonal control of organ formation in callus of Medicago sativa L. cultured in vitro. Ann J Bot 65: 654–659
Walker KA, Wendeln ML, Jaworski EG (1979) Organogenesis in callus tissue of Medicago sativa. The temporal separation of induction processes from differentiation processes. Plant Sci Lett 16: 23–30
Walton PD, Brown DCW (1988) Electrofusion of protoplasts and heterokaryon survival in the Genus Medicago. Plant Breeding 101: 137–142
Wang JW, Sorensen EL, Liang GH (1984) In vitro culture of pods from annual and perennial Medicago species. Plant Cell Rep 3: 146–148
Webb KJ (1986) Transformation of forage legumes using Agrobacterium tumefaciens. Theor Appl Genet 72: 53–58
Xu ZH, Davey MR, Cocking EC (1982) Organogenesis from root protoplasts of the forage legumes Medicago sativa and Trigonella foenum-graecum. Z Pflanzenphysiol 107: 231–235
Zagorska N, Robeva P, Dimitrov B, Shtereva R, Ganacheva V (1984) Induction of regeneration in anther cultures in Medicago sativa L. CRA Bulgare Sci 37 (8): 1099–1102
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 1990 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Arcioni, S., Damiani, F., Pezzotti, M., Lupotto, E. (1990). Alfalfa, Lucerne (Medicago spp.). In: Bajaj, Y.P.S. (eds) Legumes and Oilseed Crops I. Biotechnology in Agriculture and Forestry, vol 10. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-74448-8_10
Download citation
DOI: https://doi.org/10.1007/978-3-642-74448-8_10
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-74450-1
Online ISBN: 978-3-642-74448-8
eBook Packages: Springer Book Archive