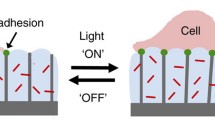
Abstract
In biomechanical and mechanobiological applications, the ability of photo-activatable materials to change properties in response to a light (photo) stimulus offers key potential advantages over other activatable materials. Not only can photo-activatable materials be used in close contact or proximity to cells and tissues without the cells or tissues being affected by the photostimulus, but photo-activatable materials also offer a level of spatiotemporal control unavailable with many other forms of smart material triggering, such as ambient heating or hydration. This chapter will give an overview of photo-activatable materials that have been developed to study cell biomechanics and mechanobiology and discuss future potential applications for these promising materials.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
D.-H. Kim, P.K. Wong, J. Park, A. Levchenko, Y. Sun, Microengineered platforms for cell mechanobiology. Annu. Rev. Biomed. Eng. 11, 203–233 (2009)
J. Wolff, Das Gesetz Der Transformation Der Knochen (A. Hirschwald, Berlin, 1891)
J.M. Mitchison, M.M. Swann, The mechanical properties of the cell surface. J. Exp. Biol. 32, 734–750 (1954)
R.M. Hochmuth, Micropipette aspiration of living cells. J. Biomech. 33, 15–22 (2000)
K.L. Sung, M.K. Kwan, F. Maldonado, W.H. Akeson, Adhesion strength of human ligament fibroblasts. J. Biomech. Eng. 116, 237–242 (1994)
M. Radmacher, Measuring the elastic properties of biological samples with the AFM. IEEE Eng. Med. Biol. Mag. 16, 47–57 (1997)
H. Haga et al., Elasticity mapping of living fibroblasts by AFM and immunofluorescence observation of the cytoskeleton. Ultramicroscopy 82, 253–258 (2000)
Y.J. Kim et al., A study of compatibility between cells and biopolymeric surfaces through quantitative measurements of adhesive forces. J. Biomater. Sci. Polym. Ed. 14, 1311–1321 (2003)
O. Thoumine, P. Kocian, A. Kottelat, J. Meister, Short-term binding of fibroblasts to fibronectin: Optical tweezers experiments and probabilistic analysis. Eur. Biophys. J. 29, 398–408 (2000)
R.L.Y. Sah et al., Biosynthetic response of cartilage explants to dynamic compression. J. Orthop. Res. 7, 619–636 (1989)
S. Noria et al., Assembly and reorientation of stress fibers drives morphological changes to endothelial cells exposed to shear stress. Am. J. Pathol. 164, 1211–1223 (2004)
R. Yoshida et al., Comb-type grafted hydrogels with rapid deswelling response to temperature changes. Nature 374, 240–242 (1995)
S. Dai, P. Ravi, K.C. Tam, pH-responsive polymers: synthesis, properties and applications. Soft Matter 4, 435 (2008)
X. Yin, A.S. Hoffman, P.S. Stayton, Poly( N-isopropylacrylamide- co -propylacrylic acid) copolymers that respond sharply to temperature and pH. Biomacromolecules 7, 1381–1385 (2006)
Y. Osada, H. Okuzaki, H. Hori, A polymer gel with electrically driven motility. Nature 355, 242–244 (1992)
S. Tasoglu et al., Guided and magnetic self-assembly of tunable magnetoceptive gels. Nat. Commun. 5, 4702 (2014)
B. Yang, W.M. Huang, C. Li, L. Li, Effects of moisture on the thermomechanical properties of a polyurethane shape memory polymer. Polymer (Guildf). 47, 1348–1356 (2006)
H. Yamaguchi et al., Photoswitchable gel assembly based on molecular recognition. Nat. Commun. 3, 603 (2012)
A. Lendlein, H. Jiang, O. Jünger, R. Langer, Light-induced shape-memory polymers. Nature 434, 879–882 (2005)
A.M. Kloxin, A.M. Kasko, C.N. Salinas, K.S. Anseth, Photodegradable hydrogels for dynamic tuning of physical and chemcial properties. Science 324, 59–63 (2009)
J. Nakanishi et al., Photoactivation of a substrate for cell adhesion under standard fluorescence microscopes. J. Am. Chem. Soc. 126, 16314–16315 (2004)
A.M. Kloxin, M.W. Tibbitt, A.M. Kasko, J.A. Fairbairn, K.S. Anseth, Tunable hydrogels for external manipulation of cellular microenvironments through controlled photodegradation. Adv. Mater. 22, 61–66 (2010)
S.J. Bryant, C.R. Nuttleman, K.S. Anseth, Cytocompatibility of UV and visible light photoinitiating systems on cultured NIH/3T3 fibroblasts in vitro. J. Biomater. Sci. Polym. Ed. 11, 439–457 (2000)
Biomaterials Science: An Introduction to Materials and Medicine. (Elsevier Academic Press, New York, 2004)
K. Han, W.-N. Yin, J.-X. Fan, F.-Y. Cao, X.-Z. Zhang, Photo-activatable substrates for site-specific differentiation of stem cells. ACS Appl. Mater. Interfaces 7, 23679–23684 (2015)
Y.-H. Gong et al., Photoresponsive ‘smart template’ via host-guest interaction for reversible cell adhesion. Macromolecules 44, 7499–7502 (2011)
D. Liu, Y. Xie, H. Shao, X. Jiang, Using azobenzene-embedded self-assembled monolayers to photochemically control cell adhesion reversibly. Angew. Chem. Int. Ed. 48, 4406–4408 (2009)
I. Tomatsu, K. Peng, A. Kros, Photoresponsive hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 63, 1257–1266 (2011)
G.D. Nicodemus, S.J. Bryant, Cell encapsulation in biodegradable hydrogels for tissue engineering applications. Tissue Eng. Part B Rev. 14, 149–165 (2008)
M.T. Frey, Y. Wang, A photo-modulatable material for probing cellular responses to substrate rigidity. Soft Matter 5, 1918–1924 (2009)
A.M. Rosales, K.M. Mabry, E.M. Nehls, K.S. Anseth, Photoresponsive elastic properties of azobenzene-containing poly(ethylene-glycol)-based hydrogels. Biomacromolecules 16, 798–806 (2015)
M. Behl, A. Lendlein, Shape-memory polymers. Mater. Today 10, 20–28 (2007)
M. Behl, M.Y. Razzaq, A. Lendlein, Multifunctional shape-memory polymers. Adv. Mater. 22, 3388–3410 (2010)
C. Liu, H. Qin, P.T. Mather, Review of progress in shape-memory polymers. J. Mater. Chem. 17, 1543 (2007)
P.T. Mather, X. Luo, I.A. Rousseau, Shape memory polymer research. Annu. Rev. Mater. Res. 39, 445–471 (2009)
A. Lendlein, R. Langer, Biodegradable, elastic shape-memory polymers for potential biomedical applications. Science 296, 1673–1676 (2002)
W. Small IV, P. Singhal, T.S. Wilson, D.J. Maitland, Biomedical applications of thermally activated shape memory polymers. J. Mater. Chem. 20, 3356–3366 (2010)
K.A. Davis, X. Luo, P.T. Mather, J.H. Henderson, Shape memory polymers for active cell culture. J. Vis. Exp. (2011). https://doi.org/10.3791/2903
R.M. Baker, J.H. Henderson, P.T. Mather, Shape memory poly(ε-caprolactone)-co-poly(ethylene glycol) foams with body temperature triggering and two-way actuation. J. Mater. Chem. B 1, 4916–4920 (2013)
L.F. Tseng, P.T. Mather, J.H. Henderson, Shape-memory-actuated change in scaffold fiber alignment directs stem cell morphology. Acta Biomater. 9, 8790–8801 (2013)
H. Lv, J. Leng, Y. Liu, S. Du, Shape-memory polymer in response to solution. Adv. Eng. Mater. 10, 592–595 (2008)
H. Koerner, G. Price, N.A. Pearce, M. Alexander, R.A. Vaia, Remotely actuated polymer nanocomposites—Stress-recovery of carbon-nanotube-filled thermoplastic elastomers. Nat. Mater. 3, 115–120 (2004)
N.G. Sahoo, Y.C. Jung, J.W. Cho, Electroactive shape memory effect of polyurethane composites filled with carbon nanotubes and conducting polymer. Mater. Manuf. Process. 22, 419–423 (2007)
D.J. Maitland, M.F. Metzger, D. Schumann, A. Lee, T.S. Wilson, Photothermal properties of shape memory polymer micro-actuators for treating stroke. Lasers Surg. Med. 30, 1–11 (2002)
W. Small IV, T.S. Wilson, W.J. Benett, J.M. Loge, D.J. Maitland, Laser-activated shape memory polymer intravascular thrombectomy device. Opt. Express 13, 8204–8213 (2005)
Q. Shou, K. Uto, M. Iwanaga, M. Ebara, T. Aoyagi, Near-infrared light-responsive shape-memory poly(ε-caprolactone) films that actuate in physiological temperature range. Polym. J. 46, 492–498 (2014)
Y. Yu, T. Ikeda, Photodeformable polymers: A new kind of promising smart material for micro- and nano-applications. Macromol. Chem. Phys. 206, 1705–1708 (2005)
A. Lendlein, M. Behl, B. Hiebl, C. Wischke, Shape-memory polymers as a technology platform for biomedical applications. Expert Rev. Med. Dev. 7, 357–379 (2010)
K.A. Davis, K.A. Burke, P.T. Mather, J.H. Henderson, Dynamic cell behavior on shape memory polymer substrates. Biomaterials 32, 2285–2293 (2011)
R.M. Baker, L.F. Tseng, M.T. Iannolo, M.E. Oest, J.H. Henderson, Self-deploying shape memory polymer scaffolds for grafting and stabilizing complex bone defects: A mouse femoral segmental defect study. Biomaterials 76, 388–398 (2016)
X. Xu et al., Shape memory RGD-containing networks: Synthesis, characterization, and application in cell culture. Macromol. Symp. 309–310, 162–172 (2011)
P. Yang, R.M. Baker, J.H. Henderson, P.T. Mather, In vitro wrinkle formation via shape memory dynamically aligns adherent cells. Soft Matter 9, 4705–4714 (2013)
R.M. Baker, M.E. Brasch, M.L. Manning, J.H. Henderson, Automated, contour-based tracking and analysis of cell behaviour over long time scales in environments of varying complexity and cell density. J. R. Soc. Interface 11, 20140386 (2014)
M. Ebara et al., Focus on the interlude between topographic transition and cell response on shape-memory surfaces. Polym. (United Kingdom) 55, 5961–5968 (2014)
T. Gong et al., The control of mesenchymal stem cell differentiation using dynamically tunable surface microgrooves. Adv. Healthc. Mater. 3, 1608–1619 (2014)
P.Y. Mengsteab et al., Spatiotemporal control of cardiac anisotropy using dynamic nanotopographic cues. Biomaterials 86, 1–10 (2016)
S.A. Turner, J. Zhou, S.S. Sheiko, V.S. Ashby, Switchable micropatterned surface topographies mediated by reversible shape memory. ACS Appl. Mater. Interfaces 6, 8017–8021 (2014)
D.M. Le, M.A. Tycon, C.J. Fecko, V.S. Ashby, Near-infrared activation of semi-crystalline shape memory polymer nanocomposites. J. Appl. Polym. Sci. 130, 4551–4557 (2013)
Q. Shou, K. Uto, W.-C. Lin, T. Aoyagi, M. Ebara, Near-infrared-irradiation-induced remote activation of surface shape-memory to direct cell orientations. Macromol. Chem. Phys. 215, 2473–2481 (2014)
C.A. Goubko, S. Majumdar, A. Basak, X. Cao, Hydrogel cell patterning incorporating photocaged RGDS peptides. Biomed. Microdev. 12, 555–568 (2010)
J. Nakanishi et al., Spatiotemporal control of cell adhesion on a self-assembled monolayer having a photocleavable protecting group. Anal. Chim. Acta 578, 100–104 (2006)
J. Nakanishi et al., Spatiotemporal control of migration of single cells on a photoactivatable cell microarray. J. Am. Chem. Soc. 129, 6694–6695 (2007)
J. Nakanishi, H. Nakayama, K. Yamaguchi, A.J. Garcia, Y. Horiike, Dynamic culture substrate that captures a specific extracellular matrix protein in response to light. Sci. Technol. Adv. Mater. 12, 44608 (2011)
Y. Kikuchi et al., Grafting poly(ethylene glycol) to a glass surface via a photocleavable linker for light-induced cell micropatterning and cell proliferation control. Chem. Lett. 37, 1062–1063 (2008)
Y. Kikuchi et al., Arraying heterotypic single cells on photoactivatable cell-culturing substrates. Langmuir 24, 13084–13095 (2008)
S. Kaneko et al., Photocontrol of cell adhesion on amino-bearing surfaces by reversible conjugation of poly(ethylene glycol) via a photocleavable linker. Phys. Chem. Chem. Phys. 13, 4051–4059 (2011)
M. Kamimura et al., Facile preparation of a photoactivatable surface on a 96-well plate: A versatile and multiplex cell migration assay platform. Phys. Chem. Chem. Phys. 17, 14159–14167 (2015)
S. Petersen et al., Phototriggering of cell adhesion by caged cyclic RGD peptides. Angew. Chem. Int. Ed. 47, 3192–3195 (2008)
J. Nakanishi et al., Precise patterning of photoactivatable glass coverslip for fluorescence observation of shape-controlled cells. Supramol. Chem. 22, 396–405 (2010)
Y. Shimizu, H. Boehm, K. Yamaguchi, J.P. Spatz, J. Nakanishi, A photoactivatable nanopatterned substrate for analyzing collective cell migration with precisely tuned cell-extracellular matrix ligand interactions. PLoS One 9(3), e91875 (2014). https://doi.org/10.1371/journal.pone.0091875
Y. Ohmuro-Matsuyama, Y. Tatsu, Photocontrolled cell adhesion on a surface functionalized with a caged arginine-glycine-aspartate peptide. Angew. Chem. Int. Ed. 47, 7527–7529 (2008)
M.D. Pierschbacher, E. Ruoslahti, Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature 309, 30–33 (1984)
G.S. Nowakowski et al., A specific heptapeptide from a phage display peptide library homes to bone marrow and binds to primitive hematopoietic stem cells. Stem Cells 22, 1030–1038 (2004)
F.-Y. Cao, W.-N. Yin, J.-X. Fan, R.-X. Zhuo, X.-Z. Zhang, A novel function of BMHP1 and cBMHP1 peptides to induce the osteogenic differentiation of mesenchymal stem cells. Biomater. Sci. 3, 345–351 (2015)
M.W. Tibbitt, A.M. Kloxin, K.U. Dyamenahalli, K.S. Anseth, Controlled two-photon photodegradation of PEG hydrogels to study and manipulate subcellular interactions on soft materials. Soft Matter 6, 5100 (2010)
B. Wildt, D. Wirtz, P.C. Searson, Programmed subcellular release for studying the dynamics of cell detachment. Nat. Methods 6, 211–213 (2009)
A.J. Ridley et al., Cell migration: Integrating signals from front to back. Science 302, 1704–1709 (2003)
P. Friedl, B. Weigelin, Interstitial leukocyte migration and immune function. Nat. Immunol. 9, 960–969 (2008)
P.L. Ryan, R.A. Foty, J. Kohn, M.S. Steinberg, Tissue spreading on implantable substrates is a competitive outcome of cell-cell vs. cell-substratum adhesivity. Proc. Natl. Acad. Sci. 98, 4323–4327 (2001)
J. Bourget, M. Guillemette, T. Veres, F.A. Auger, L. Germain, Alignment of cells and extracellular matrix within tissue-engineered substitutes. Adv. Biomater. Sci. Biomed. Appl. Ref., 365–390 (2013). https://doi.org/10.5772/54142
C.M. Kirschner, D.L. Alge, S.T. Gould, K.S. Anseth, Clickable, photodegradable hydrogels to dynamically modulate valvular interstitial cell phenotype. Adv. Healthc. Mater. 3, 649–657 (2014)
F. Guilak et al., Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell 5, 17–26 (2009)
S. Tavella et al., Regulated expression of fibronectin, laminin and related integrin receptors during the early chondrocyte differentiation. J. Cell Sci. 110, 2261–2270 (1997)
A.M. Kloxin, J.A. Benton, K.S. Anseth, In situ elasticity modulation with dynamic substrates to direct cell phenotype. Biomaterials 31, 1–8 (2010)
H. Wang, S.M. Haeger, A.M. Kloxin, L.A. Leinwand, K.S. Anseth, Redirecting valvular myofibroblasts into dormant fibroblasts through light-mediated reduction in substrate modulus. PLoS One 7 (2012)
C. Yang, M.W. Tibbitt, L. Basta, K.S. Anseth, Mechanical memory and dosing influence stem cell fate. Nat. Mater. 13, 645–652 (2014)
S. Dupont et al., Role of YAP/TAZ in mechanotransduction. Nature 474, 179–183 (2011)
G. Halder, S. Dupont, S. Piccolo, Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nat. Publ. Gr. 13, 591–600 (2012)
M.J. Salierno, A.J. Garcia, A. Del Campo, Photo-activatable surfaces for cell migration assays. Adv. Funct. Mater. 23, 5974–5980 (2013)
R.M. Pope, E.S. Fry, Absorption spectrum (340–640 nm) of pure water. I. Photothermal measurement. Appl. Opt. 36, 8710–8723 (1997)
H. Zhang, H. Xia, Y. Zhao, Optically triggered and spatially controllable shape-memory polymer–gold nanoparticle composite materials. J. Mater. Chem. 22, 845–849 (2012)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Pede, M.E., Henderson, J.H. (2018). The Use of Photo-Activatable Materials for the Study of Cell Biomechanics and Mechanobiology. In: Van Hoorick, J., Ottevaere, H., Thienpont, H., Dubruel, P., Van Vlierberghe, S. (eds) Polymer and Photonic Materials Towards Biomedical Breakthroughs. Micro- and Opto-Electronic Materials, Structures, and Systems. Springer, Cham. https://doi.org/10.1007/978-3-319-75801-5_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-75801-5_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-75800-8
Online ISBN: 978-3-319-75801-5
eBook Packages: EnergyEnergy (R0)